The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome
- PMID: 17160906
- PMCID: PMC1785313
- DOI: 10.1086/510499
The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome
Abstract
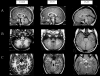
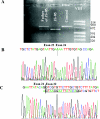
Joubert syndrome (JS) is an autosomal recessive disorder characterized by cerebellar vermis hypoplasia associated with hypotonia, developmental delay, abnormal respiratory patterns, and abnormal eye movements. The association of retinal dystrophy and renal anomalies defines JS type B. JS is a genetically heterogeneous condition with mutations in two genes, AHI1 and CEP290, identified to date. In addition, NPHP1 deletions identical to those that cause juvenile nephronophthisis have been identified in a subset of patients with a mild form of cerebellar and brainstem anomaly. Occipital encephalocele and/or polydactyly have occasionally been reported in some patients with JS, and these phenotypic features can also be observed in Meckel-Gruber syndrome (MKS). MKS is a rare, autosomal recessive lethal condition characterized by central nervous system malformations (typically, occipital meningoencephalocele), postaxial polydactyly, multicystic kidney dysplasia, and ductal proliferation in the portal area of the liver. Since there is obvious phenotypic overlap between JS and MKS, we hypothesized that mutations in the recently identified MKS genes, MKS1 on chromosome 17q and MKS3 on 8q, may be a cause of JS. After mutation analysis of MKS1 and MKS3 in a series of patients with JS (n=22), we identified MKS3 mutations in four patients with JS, thus defining MKS3 as the sixth JS locus (JBTS6). No MKS1 mutations were identified in this series, suggesting that the allelism is restricted to MKS3.
Figures

References
Web Resources
-
- Online Mendelian Inheritance in Man (OMIM),http://www.ncbi.nlm.nih.gov/Omim/ (for JS, MKS, and BBS) - PubMed
-
- PolyPhen, http://genetics.bwh.harvard.edu/pph/
-
- Splice Site Prediction by SSF, http://www.umd.be/SSF
-
- TMHMM Server, http://www.cbs.dtu.dk/services/TMHMM/
References
-
- Joubert M, Eisenring JJ, Andermann F (1968) Familial dysgenesis of the vermis: a syndrome of hyperventilation, abnormal eye movements and retardation. Neurology 18:302–303 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

