Rapid neuroadaptation in the nucleus accumbens and bed nucleus of the stria terminalis mediates suppression of operant responding during withdrawal from acute opioid dependence
- PMID: 17161915
- PMCID: PMC1805631
- DOI: 10.1016/j.neuroscience.2006.11.002
Rapid neuroadaptation in the nucleus accumbens and bed nucleus of the stria terminalis mediates suppression of operant responding during withdrawal from acute opioid dependence
Abstract
Single injections of morphine induce a state of acute opioid dependence in humans and animals, measured as precipitated withdrawal when an antagonist is administered 4-24 h after morphine. Additional morphine exposure at daily or weekly intervals results in further increases in withdrawal severity, suggesting that acute opioid dependence reflects the early stages in the development of a chronic state of dependence. The current study evaluated the role of the nucleus accumbens (NAC), bed nucleus of stria terminalis (BNST), interstitial nucleus of posterior limb of the anterior commissure (IPAC), and central amygdala (CeA) in the expression of antagonist-precipitated suppression of operant responding for food as a measure of withdrawal from acute opioid dependence. Rats trained on a fixed-ratio 15 schedule received one or four daily injections of morphine, with the lipophobic opioid antagonist methylnaloxonium (16-2000 ng) infused into one of the brain regions or the lateral ventricle (i.c.v.) 4 h after the final morphine injection. After acute morphine methylnaloxonium was more potent upon infusion into the NAC (17.9-fold potency shift), BNST (6.8-fold) and CeA (5.5-fold) than it was upon i.c.v. administration. Following repeat morphine the NAC and BNST but not CeA continued to show greater sensitivity relative to i.c.v. infusion (12.9-, 8.7-, and 3.2-fold potency shifts, respectively). The IPAC was insensitive to methylnaloxonium after acute or repeat morphine at doses that reliably suppressed responding upon i.c.v. infusion (125-500 ng). Thus, among the components of extended amygdala examined in this study, rapid neuroadaptation within the nucleus accumbens and bed nucleus of the stria terminalis appear to play the most prominent role in antagonist-precipitated suppression of operant responding during the early stages in the development of opioid dependence.
Figures
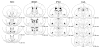


References
-
- Adams JU, Holtzman SG. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther. 1990;253:483–489. - PubMed
-
- Adams JU, Holtzman SG. Naltrexone-sensitizing effects of centrally administered morphine and opioid peptides. Eur J Pharmacol. 1991;193:67–73. - PubMed
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. - PubMed
-
- Azorlosa JL, Stitzer ML, Greenwald MK. Opioid physical dependence development: effect of single versus repeated morphine pretreatments and of subjects' opioid exposure history. Psychopharmacology (Berl) 1994;114:71–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

