Synthesis and biological activation of an ethylene glycol-linked amino acid conjugate of cyclic cidofovir
- PMID: 17161946
- PMCID: PMC1899532
- DOI: 10.1016/j.bmcl.2006.11.012
Synthesis and biological activation of an ethylene glycol-linked amino acid conjugate of cyclic cidofovir
Abstract
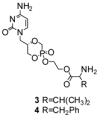
Cidofovir (HPMPC) is a broad-spectrum anti-viral agent whose potential, particularly in biodefense scenarios, is limited by its low oral bioavailability. Two prodrugs (3 and 4) created by conjugating ethylene glycol-linked amino acids (L-Val, L-Phe) with the cyclic form of cidofovir (cHPMPC) via a P-O ester bond were synthesized and their pH-dependent stability (3 and 4), potential for in vivo reconversion to drug (3), and oral bioavailability (3) were evaluated. The prodrugs were stable in buffer between pH 3 and 5, but underwent rapid hydrolysis in liver (t(1/2) = 3.7 min), intestinal (t(1/2) = 12.5 min), and Caco-2 cell homogenates (t(1/2) = 20.2 min). In vivo (rat), prodrug 3 was >90% reconverted to cHPMPC. The prodrug was 4x more active than ganciclovir (IC50 value, 0.68 microM vs 3.0 microM) in a HCMV plaque reduction assay. However, its oral bioavailability in a rat model was similar to the parent drug. The contrast between the promising activation properties and unenhanced transport of the prodrug is briefly discussed.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

