Fluorogenic label for biomolecular imaging
- PMID: 17163679
- PMCID: PMC2862228
- DOI: 10.1021/cb600132m
Fluorogenic label for biomolecular imaging
Abstract
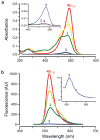
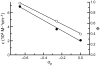
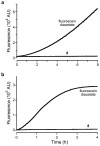
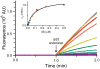
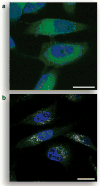
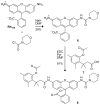
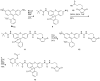
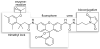
Traditional small-molecule fluorophores are always fluorescent. This attribute can obscure valuable information in biological experiments. Here, we report on a versatile "latent" fluorophore that overcomes this limitation. At the core of the latent fluorophore is a derivative of rhodamine in which one nitrogen is modified as a urea. That modification enables rhodamine to retain half of its fluorescence while facilitating conjugation to a target molecule. The other nitrogen of rhodamine is modified with a "trimethyl lock", which enables fluorescence to be unmasked fully by a single user-designated chemical reaction. An esterase-reactive latent fluorophore was synthesized in high yield and attached covalently to a cationic protein. The resulting conjugate was not fluorescent in the absence of esterases. The enzymatic activity of esterases in endocytic vesicles and the cytosol educed fluorescence, enabling the time-lapse imaging of endocytosis into live human cells and thus providing unprecedented spatiotemporal resolution of this process. The modular design of this "fluorogenic label" enables the facile synthesis of an ensemble of small-molecule probes for the illumination of numerous biochemical and cell biological processes.
Figures

References
-
- Haugland RP, Spence MTZ, Johnson ID, Basey A. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies. 10. Molecular Probes; Eugene, OR: 2005.
-
- Chandran SS, Dickson KA, Raines RT. Latent fluorophore based on the trimethyl lock. J Am Chem Soc. 2005;127:1652–1653. - PubMed
-
- Borchardt RT, Cohen LA. Stereopopulation control. II. Rate enhancement of intramolecular nucleophilic displacement. J Am Chem Soc. 1972;94:9166–9174. - PubMed
-
- Milstein S, Cohen LA. Stereopopulation control. I. Rate enhancement in the lactonizations of o-hydroxyhydrocinnamic acids. J Am Chem Soc. 1972;94:9158–9165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

