Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis
- PMID: 17163990
- PMCID: PMC1794528
- DOI: 10.1186/ar2093
Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis
Abstract
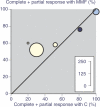
Mycophenolate mofetil (MMF) is an immunosuppressant drug being used for induction and maintenance of remission of lupus nephritis in systemic lupus erythematosus. Evidence about its use was sought from full publications and abstracts of randomised trials and cohort studies by using a variety of search strategies. Efficacy and adverse event outcomes were sought. Five randomised trials enrolled patients with World Health Organization (WHO) class III, IV, or V (mostly IV) lupus nephritis, predominantly comparing MMF (1 to 3 g daily) with cyclophosphamide and steroid. Complete response and complete or partial response was significantly more frequent with MMF than with cyclophosphamide, with numbers needed to treat of 8 (95% confidence interval 4.3 to 60) to induce one additional complete or partial response, with wide confidence intervals. Death was reported less frequently with MMF (0.7%, 1 death in 152 patients) than with cyclophosphamide (7.8%, 12 deaths in 154 patients), with a number needed to treat to prevent (NNTp) one death of 14 (8 to 48). Hospital admission was also lower with MMF (1.7% versus 15%; NNTp 7.4 [4.8 to 16]). Serious infections, leucopaenia, amenorrhoea, and hair loss were all significantly less frequent with MMF than with cyclophosphamide, but diarrhoea was significantly more common with MMF. Ten of 18 cohort studies enrolled only patients with lupus nephritis (author-defined or WHO class III to V). Seven of these 10 reported that complete or partial response with MMF (mostly 1 or 2 g daily) with steroid occurred in 121/151 (80%) and that treatment failure or no response occurred in 30/151 (20%). Adverse events were generally similar in cohort studies with and without only patients with lupus nephritis. In all 18 cohorts, gastrointestinal adverse events (diarrhoea, nausea, vomiting) occurred in 30%, infection in 23%, and serious infection in 4.3%. Adverse event discontinuations occurred in 14% and lack of efficacy occurred in 10%. There was a single death with MMF, a mortality rate over the course of 1 year of approximately 0.2%. The results form a basis on which to plan future studies and provide a guide for the use of MMF in lupus nephritis until results of larger studies are available. At least one such study is under way.
Figures

References
-
- Nived O, Sturfelt G, Wollheim F. Systemic lupus erythematosus in an adult population in southern Sweden: incidence, prevalence and validity of ARA revised classification criteria. Br J Rheumatol. 1985;24:147–154. - PubMed
-
- Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Arthritis Rheum. 1995;38:551–558. - PubMed
-
- Hopkinson ND, Doherty M, Powell RJ. The prevalence and incidence of systemic lupus erythematosus in Nottingham, UK, 1989–1990. Br J Rheumatol. 1993;32:110–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

