The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma
- PMID: 17164332
- PMCID: PMC1748251
- DOI: 10.1073/pnas.0609329103
The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma
Abstract
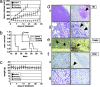
A critical role for vascular endothelial factor (VEGF) has been demonstrated in multiple myeloma (MM) pathogenesis. Here, we characterized the effect of the small-molecule VEGF receptor inhibitor pazopanib on MM cells in the bone marrow milieu. Pazopanib inhibits VEGF-triggered signaling pathways in both tumor and endothelial cells, thereby blocking in vitro MM cell growth, survival, and migration, and inhibits VEGF-induced up-regulation of adhesion molecules on both endothelial and tumor cells, thereby abrogating endothelial cell-MM cell binding and associated cell proliferation. We show that pazopanib is the first-in-class VEGF receptor inhibitor to inhibit in vivo tumor cell growth associated with increased MM cell apoptosis, decreased angiogenesis, and prolonged survival in a mouse xenograft model of human MM. Low-dose pazopanib demonstrates synergistic cytotoxicity with conventional (melphalan) and novel (bortezomib and immunomodulatory drugs) therapies. Finally, gene expression and signaling network analysis show transcriptional changes of several cancer-related genes, in particular c-Myc. Using siRNA, we confirm the role of c-Myc in VEGF production and secretion, as well as angiogenesis. These preclinical studies provide the rationale for clinical evaluation of pazopanib, alone and in combination with conventional and novel therapies, to increase efficacy, overcome drug resistance, reduce toxicity, and improve patient outcome in MM.
Conflict of interest statement
Conflict of interest statement: R.K. and L.N.P. are employees of GlaxoSmithKline, Research Triangle Park, NC.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

