Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development
- PMID: 17164422
- PMCID: PMC2424197
- DOI: 10.1242/dev.02701
Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development
Abstract
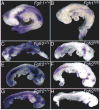
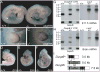
Mitogen-activated protein kinase (MAPK) pathways are major mediators of extracellular signals that are transduced to the nucleus. MAPK signaling is attenuated at several levels, and one class of dual-specificity phosphatases, the MAPK phosphatases (MKPs), inhibit MAPK signaling by dephosphorylating activated MAPKs. Several of the MKPs are themselves induced by the signaling pathways they regulate, forming negative feedback loops that attenuate the signals. We show here that in mouse embryos, Fibroblast growth factor receptors (FGFRs) are required for transcription of Dusp6, which encodes MKP3, an extracellular signal-regulated kinase (ERK)-specific MKP. Targeted inactivation of Dusp6 increases levels of phosphorylated ERK, as well as the pERK target, Erm, and transcripts initiated from the Dusp6 promoter itself. Finally, the Dusp6 mutant allele causes variably penetrant, dominant postnatal lethality, skeletal dwarfism, coronal craniosynostosis and hearing loss; phenotypes that are also characteristic of mutations that activate FGFRs inappropriately. Taken together, these results show that DUSP6 serves in vivo as a negative feedback regulator of FGFR signaling and suggest that mutations in DUSP6 or related genes are candidates for causing or modifying unexplained cases of FGFR-like syndromes.
Figures




Similar articles
-
Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter.Biochem J. 2008 Jun 1;412(2):287-98. doi: 10.1042/BJ20071512. Biochem J. 2008. PMID: 18321244 Free PMC article.
-
Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos.Curr Biol. 2003 Jun 17;13(12):1009-18. doi: 10.1016/s0960-9822(03)00381-6. Curr Biol. 2003. PMID: 12814546
-
Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite.Development. 2005 Mar;132(6):1305-14. doi: 10.1242/dev.01699. Epub 2005 Feb 16. Development. 2005. PMID: 15716340
-
The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs).Semin Cell Dev Biol. 2016 Feb;50:125-32. doi: 10.1016/j.semcdb.2016.01.009. Epub 2016 Jan 11. Semin Cell Dev Biol. 2016. PMID: 26791049 Free PMC article. Review.
-
Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases.Oncogene. 2007 May 14;26(22):3203-13. doi: 10.1038/sj.onc.1210412. Oncogene. 2007. PMID: 17496916 Review.
Cited by
-
Phosphorylation and stabilization of Arabidopsis MAP kinase phosphatase 1 in response to UV-B stress.J Biol Chem. 2013 Jan 4;288(1):480-6. doi: 10.1074/jbc.M112.434654. Epub 2012 Nov 27. J Biol Chem. 2013. PMID: 23188831 Free PMC article.
-
Cranial sutures: a multidisciplinary review.Childs Nerv Syst. 2013 Jun;29(6):893-905. doi: 10.1007/s00381-013-2061-4. Epub 2013 Mar 8. Childs Nerv Syst. 2013. PMID: 23471493 Review.
-
Central Roles and Regulatory Mechanisms of Dual-Specificity MAPK Phosphatases in Developmental and Stress Signaling.Front Plant Sci. 2018 Nov 20;9:1697. doi: 10.3389/fpls.2018.01697. eCollection 2018. Front Plant Sci. 2018. PMID: 30515185 Free PMC article. Review.
-
New insight on FGFR3-related chondrodysplasias molecular physiopathology revealed by human chondrocyte gene expression profiling.PLoS One. 2009 Oct 29;4(10):e7633. doi: 10.1371/journal.pone.0007633. PLoS One. 2009. PMID: 19898608 Free PMC article.
-
Dynamic changes in cis-regulatory occupancy by Six1 and its cooperative interactions with distinct cofactors drive lineage-specific gene expression programs during progressive differentiation of the auditory sensory epithelium.Nucleic Acids Res. 2020 Apr 6;48(6):2880-2896. doi: 10.1093/nar/gkaa012. Nucleic Acids Res. 2020. PMID: 31956913 Free PMC article.
References
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. - PubMed
-
- Brodie SG, Deng CX. Mouse models orthologous to FGFR3-related skeletal dysplasias. Pediatr Pathol Mol Med. 2003;22:87–103. - PubMed
-
- Bundschu K, Knobeloch KP, Ullrich M, Schinke T, Amling M, Engelhardt CM, Renné T, Walter U, Schuh K. Gene disruption of Spred-2 causes dwarfism. J Biol Chem. 2005;280:28572–28580. - PubMed
-
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. Faseb J. 2000;14:6–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

