Supramolecular allosteric cofacial porphyrin complexes
- PMID: 17165783
- PMCID: PMC2525615
- DOI: 10.1021/ja0661010
Supramolecular allosteric cofacial porphyrin complexes
Abstract
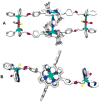
Nature routinely uses cooperative interactions to regulate cellular activity. For years, chemists have designed synthetic systems that aim toward harnessing the reactivity common to natural biological systems. By learning how to control these interactions in situ, one begins to allow for the preparation of man-made biomimetic systems that can efficiently mimic the interactions found in Nature. To this end, we have designed a synthetic protocol for the preparation of flexible metal-directed supramolecular cofacial porphyrin complexes which are readily obtained in greater than 90% yield through the use of new hemilabile porphyrin ligands with bifunctional ether-phosphine or thioether-phosphine substituents at the 5 and 15 positions on the porphyrin ring. The resulting architectures contain two hemilabile ligand-metal domains (RhI or CuI sites) and two cofacially aligned porphyrins (ZnII sites), offering orthogonal functionalities and allowing these multimetallic complexes to exist in two states, "condensed" or "open". Combining the ether-phosphine ligand with the appropriate RhI or CuI transition-metal precursors results in "open" macrocyclic products. In contrast, reacting the thioether-phosphine ligand with RhI or CuI precursors yields condensed structures that can be converted into their "open" macrocyclic forms via introduction of additional ancillary ligands. The change in cavity size that occurs allows these structures to function as allosteric catalysts for the acyl transfer reaction between X-pyridylcarbinol (where X = 2, 3, or 4) and 1-acetylimidazole. For 3- and 4-pyridylcarbinol, the "open" macrocycle accelerates the acyl transfer reaction more than the condensed analogue and significantly more than the porphyrin monomer. In contrast, an allosteric effect was not observed for 2-pyridylcarbinol, which is expected to be a weaker binder and is unfavorably constrained inside the macrocyclic cavity.
Figures



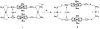


Similar articles
-
Heteroligated supramolecular coordination complexes formed via the halide-induced ligand rearrangement reaction.Acc Chem Res. 2008 Dec;41(12):1618-29. doi: 10.1021/ar800025w. Acc Chem Res. 2008. PMID: 18642933 Free PMC article.
-
Metallomacrocycles that incorporate cofacially aligned diimide units.Chem Asian J. 2006 Nov 20;1(5):686-92. doi: 10.1002/asia.200600205. Chem Asian J. 2006. PMID: 17441109
-
A convergent coordination chemistry-based approach to dissymmetric macrocyclic cofacial porphyrin complexes.Inorg Chem. 2007 Sep 17;46(19):7716-8. doi: 10.1021/ic701424j. Epub 2007 Aug 14. Inorg Chem. 2007. PMID: 17696341
-
Bioinspired Applications of Porphyrin Derivatives.Acc Chem Res. 2021 May 4;54(9):2249-2260. doi: 10.1021/acs.accounts.1c00114. Epub 2021 Apr 23. Acc Chem Res. 2021. PMID: 33891405 Review.
-
Boron complexes of porphyrins and related polypyrrole ligands: unexpected chemistry for both boron and the porphyrin.Chem Commun (Camb). 2008 May 14;(18):2090-102. doi: 10.1039/b714894a. Epub 2008 Feb 5. Chem Commun (Camb). 2008. PMID: 18438481 Review.
Cited by
-
Size-Selective Hydroformylation by a Rhodium Catalyst Confined in a Supramolecular Cage.Chemistry. 2019 Jan 7;25(2):609-620. doi: 10.1002/chem.201804333. Epub 2018 Dec 11. Chemistry. 2019. PMID: 30351486 Free PMC article.
-
Heteroleptic metallosupramolecular aggregates/complexation for supramolecular catalysis.Beilstein J Org Chem. 2022 May 27;18:597-630. doi: 10.3762/bjoc.18.62. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35673407 Free PMC article. Review.
-
Design, synthesis and applications of responsive macrocycles.Commun Chem. 2020 Dec 17;3(1):189. doi: 10.1038/s42004-020-00438-2. Commun Chem. 2020. PMID: 36703444 Free PMC article. Review.
-
Reversible Ligand Pairing and Sorting Processes Leading to Heteroligated Palladium(II) Complexes with Hemilabile Ligands.Organometallics. 2009 Feb 23;28(4):1068-1074. doi: 10.1021/om801060m. Epub 2009 Jan 27. Organometallics. 2009. PMID: 34446977 Free PMC article.
-
How to not build a cage: endohedral functionalization of polyoxometalate-based metal-organic polyhedra.Chem Sci. 2021 Apr 3;12(21):7361-7368. doi: 10.1039/d1sc01243f. Chem Sci. 2021. PMID: 34163825 Free PMC article.
References
-
- Cowan JA, Sanders JKM. J Chem Soc, Chem Commun. 1985:1213–14.
-
- Fletcher JT, Therien MJ. J Am Chem Soc. 2000;122:12393–12394.
-
- Collman JP, Bencosme CS, Durand RR, Jr, Kreh RP, Anson FC. J Am Chem Soc. 1983;105:2699–703.
-
- Feiters MC, Fyfe MCT, Martinez-Diaz MV, Menzer S, Nolte RJM, Stoddart JF, van Kan PJM, Williams DJ. J Am Chem Soc. 1997;119:8119–8120.
-
- Yagi S, Yonekura I, Awakura M, Ezoe M, Takagishi T. Chem Commun. 2001:557–558.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

