Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells
- PMID: 17166093
- PMCID: PMC1828885
- DOI: 10.1042/BJ20061617
Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells
Abstract
The 5-lipoxygenase product 5-oxo-ETE (5-oxo-eicosatetraenoic acid) is a highly potent granulocyte chemoattractant that is synthesized from 5-HETE (5-hydroxyeicosatetraenoic acid) by 5-HEDH (5-hydroxyeicosanoid dehydrogenase). In the present study, we found that 5-HEDH activity is induced in U937 monocytic cells by differentiation towards macrophages with PMA and in HL-60 myeloblastic cells by 1,25-dihydroxy-vitamin D3. We used PMA-differentiated U937 cells to investigate further the regulation of 5-HEDH. This enzyme exhibits approx. 10000-fold selectivity for NADP+ over NAD+ as a cofactor for the oxidation of 5-HETE, which is maximal at pH 10.2. In contrast, the reverse reaction (5-oxo-ETE-->5-HETE) is NADPH-dependent and is maximal at pH 6. Although the K(m) for the forward reaction (670 nM) is about twice that for the reverse reaction at neutral pH, the V(max) is approx 8-fold higher. The oxidation of 5-HETE to 5-oxo-ETE is supported by very low concentrations of NADP(+) (K(m) 139 nM), inhibited by NADPH (K(i) 224 nM) and is consistent with a ping-pong mechanism. The amount of 5-oxo-ETE synthesized by 5-HEDH depends on the ratio of NADP+ to NADPH. Exposure of U937 cells to oxidative stress (t-butyl hydroperoxide) increased the ratio of NADP+ to NADPH from approx. 0.08 in resting cells to approx. 3, and this was accompanied by a dramatic increase in 5-HETE oxidation to 5-oxo-ETE. We conclude that differentiation of monocytic cells towards macrophages results in enhanced 5-oxo-ETE synthesis and that the ability of cells to synthesize 5-oxo-ETE is tightly regulated by the ratio of intracellular NADP+ to NADPH.
Figures

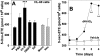





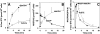

Similar articles
-
Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells.J Biol Chem. 2004 Sep 24;279(39):40376-84. doi: 10.1074/jbc.M401294200. Epub 2004 Jul 2. J Biol Chem. 2004. PMID: 15234979
-
Substrate selectivity of 5-hydroxyeicosanoid dehydrogenase and its inhibition by 5-hydroxy-Delta6-long-chain fatty acids.J Pharmacol Exp Ther. 2009 Apr;329(1):335-41. doi: 10.1124/jpet.108.143453. Epub 2009 Jan 22. J Pharmacol Exp Ther. 2009. PMID: 19164464 Free PMC article.
-
Airway epithelial cells synthesize the lipid mediator 5-oxo-ETE in response to oxidative stress.Free Radic Biol Med. 2007 Mar 1;42(5):654-64. doi: 10.1016/j.freeradbiomed.2006.12.006. Epub 2006 Dec 14. Free Radic Biol Med. 2007. PMID: 17291989 Free PMC article.
-
The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor.Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Prog Lipid Res. 2013. PMID: 24056189 Free PMC article. Review.
-
5-Oxo-ETE and the OXE receptor.Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):98-104. doi: 10.1016/j.prostaglandins.2009.05.002. Epub 2009 May 18. Prostaglandins Other Lipid Mediat. 2009. PMID: 19450703 Free PMC article. Review.
Cited by
-
Wound redox gradients revisited.Semin Cell Dev Biol. 2018 Aug;80:13-16. doi: 10.1016/j.semcdb.2017.07.038. Epub 2017 Jul 24. Semin Cell Dev Biol. 2018. PMID: 28751250 Free PMC article. Review.
-
Biosynthesis and actions of 5-oxoeicosatetraenoic acid (5-oxo-ETE) on feline granulocytes.Biochem Pharmacol. 2015 Aug 1;96(3):247-55. doi: 10.1016/j.bcp.2015.05.009. Epub 2015 May 29. Biochem Pharmacol. 2015. PMID: 26032638 Free PMC article.
-
Image-Based Measurement of H2O2 Reaction-Diffusion in Wounded Zebrafish Larvae.Biophys J. 2017 May 9;112(9):2011-2018. doi: 10.1016/j.bpj.2017.03.021. Biophys J. 2017. PMID: 28494970 Free PMC article.
-
Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids.Annu Rev Physiol. 2014;76:79-105. doi: 10.1146/annurev-physiol-021113-170341. Epub 2013 Oct 16. Annu Rev Physiol. 2014. PMID: 24161076 Free PMC article. Review.
-
Signaling actions of electrophiles: anti-inflammatory therapeutic candidates.Mol Interv. 2010 Feb;10(1):39-50. doi: 10.1124/mi.10.1.7. Mol Interv. 2010. PMID: 20124562 Free PMC article. Review.
References
-
- Funk C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. - PubMed
-
- Powell W. S., Chung D., Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J. Immunol. 1995;154:4123–4132. - PubMed
-
- Powell W. S., Ahmed S., Gravel S., Rokach J. Eotaxin and RANTES enhance 5-oxo-6,8,11,14-eicosatetraenoic acid-induced eosinophil chemotaxis. J. Allergy Clin. Immunol. 2001;107:272–278. - PubMed
-
- Czech W., Barbisch M., Tenscher K., Schopf E., Schröder J. M., Norgauer J. Chemotactic 5-oxo-eicosatetraenoic acids induce oxygen radical production, Ca2+-mobilization, and actin reorganization in human eosinophils via a pertussis toxin-sensitive G-protein. J. Invest. Dermatol. 1997;108:108–112. - PubMed
-
- O'Flaherty J. T., Kuroki M., Nixon A. B., Wijkander J., Yee E., Lee S. L., Smitherman P. K., Wykle R. L., Daniel L. W. 5-Oxo-eicosatetraenoate is a broadly active, eosinophil- selective stimulus for human granulocytes. J. Immunol. 1996;157:336–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

