The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months
- PMID: 17166221
- PMCID: PMC2063969
- DOI: 10.1111/j.1471-0528.2006.01165.x
The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months
Abstract
Objective: To assess the long-term effects of in utero exposure to magnesium sulphate for children whose mothers had pre-eclampsia.
Design: Assessment at 18 months of age for children whose mothers were recruited to the Magpie Trial (recruitment 1998-2001 ISRCTN 86938761), which compared magnesium sulphate with placebo.
Setting: Follow-up of children born at 125 centres in 19 countries across five continents.
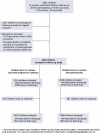
Population: A total of 6922 children were born to women randomised before delivery at follow-up centres. Of these, 2271 were not included for logistic reasons and 168 were excluded (101 at a centre where <20% were contacted, 40 whose death or disability was due to a problem at conception or embryogenesis and 27 whose parent/s opted out). Therefore, 4483 children were included in follow-up, of whom 3283 (73%) were contacted.
Methods: Assessment by questionnaire, with interview and neurodevelopmental testing of selected children.
Main outcome measures: Death or neurosensory disability at age of 18 months.
Results: Of those allocated magnesium sulphate, 245/1635 (15.0%) were dead or had neurosensory disability at 18 months compared with 233/1648 (14.1%) allocated placebo (relative risk [RR] 1.06, 95% CI 0.90-1.25), and of survivors, 19/1409 (1.3%) had neurosensory disability at 18 months compared with 27/1442 (1.9%) (RR 0.72, 95% CI 0.40-1.29). There were no substantial differences in causes of death or in the risk of individual impairments or disabilities.
Conclusions: The lower risk of eclampsia following prophylaxis with magnesium sulphate was not associated with a clear difference in the risk of death or disability for children at 18 months.
Figures
References
-
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80–3. - PubMed
-
- The National Institute for Clinical Excellence, The Scottish Executive Health Department, The Department of Health Social Services and Public Safety Northern Ireland. Why Mothers Die 1997–1999: The Fifth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press; 2001.
-
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547–53. - PubMed
-
- Department of Health. Confidential Enquiry into Stillbirths and Deaths in Infancy. London: Department of Health; 1996.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical