Differential involvement of endogenous opioids in sucrose consumption and food reinforcement
- PMID: 17166571
- PMCID: PMC1868438
- DOI: 10.1016/j.pbb.2006.10.015
Differential involvement of endogenous opioids in sucrose consumption and food reinforcement
Abstract
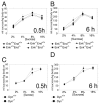
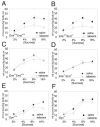
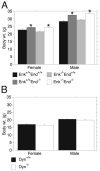
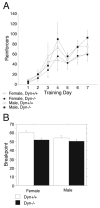
Endogenous opioids within the central nervous system are postulated to mediate hedonic aspects of feeding behavior. To identify the relevant endogenous opioid receptor ligands, mice lacking one or two of the opioid peptide families beta-endorphin, enkephalins or dynorphins were tested for sucrose preference in a two-bottle free-choice drinking paradigm under drug-naïve conditions and following treatment with an opioid antagonist (1 mg/kg naloxone i.p.) or saline. Basal sucrose consumption was unaltered in all of the knockout genotypes compared to their congenic wild-type C57BL/6 littermates during 0.5 and 6 h access to a bottle containing 2, 4, 8, or 16% sucrose and a second bottle containing water. Moreover, all mutant genotypes and wildtype mice exhibited a similar compensatory decrease in overnight food intake following the extra caloric load from 6 h sucrose access. Although these basal responses to sucrose were unaffected by the knockout genotypes, naloxone reduced sucrose consumption by 50% compared to saline treatment during the first 0.5 h in wild-type and beta-endorphin knockout mice, but had no effect in enkephalin knockouts, beta-endorphin and enkephalin double knockouts, or dynorphin knockouts. These data suggest that naloxone reduces sucrose consumption in wild-type mice by blocking endogenous enkephalin and/or dynorphin signaling, but not beta-endorphin. Dynorphin knockouts in the current study had bar-pressing responses for a palatable food reinforcer in an operant procedure under free-feeding conditions similar to wild-type mice while we found in a previous study that beta-endorphin and enkephalin knockout mice had reduced motivation to respond [Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking beta-endorphin and enkephalin. J Neurosci 2002;22:8251-8258.]. We conclude from these studies directly comparing three strains of opioid peptide knockout mice that enkephalin and dynorphin can modulate sucrose preference but are not necessary to support sucrose consumption. However, dynorphin was not necessary to support wildtype levels of operant responding suggesting that only enkephalin and beta-endorphin modulate conditioned food reinforcement.
Figures

Similar articles
-
Selective reward deficit in mice lacking beta-endorphin and enkephalin.J Neurosci. 2002 Sep 15;22(18):8251-8. doi: 10.1523/JNEUROSCI.22-18-08251.2002. J Neurosci. 2002. PMID: 12223579 Free PMC article.
-
The contribution of endogenous opioids to food reward is dependent on sex and background strain.Neuroscience. 2007 Jan 5;144(1):17-25. doi: 10.1016/j.neuroscience.2006.08.067. Epub 2006 Oct 13. Neuroscience. 2007. PMID: 17049174
-
Operant self-administration of ethanol in C57BL/6 mice lacking beta-endorphin and enkephalin.Pharmacol Biochem Behav. 2004 Sep;79(1):171-81. doi: 10.1016/j.pbb.2004.07.002. Pharmacol Biochem Behav. 2004. PMID: 15388297
-
Endogenous opiates and behavior: 2012.Peptides. 2013 Dec;50:55-95. doi: 10.1016/j.peptides.2013.10.001. Epub 2013 Oct 12. Peptides. 2013. PMID: 24126281 Review.
-
Endogenous opiates and behavior: 2014.Peptides. 2016 Jan;75:18-70. doi: 10.1016/j.peptides.2015.10.009. Epub 2015 Nov 10. Peptides. 2016. PMID: 26551874 Review.
Cited by
-
Arcuate Kisspeptin Neurons Coordinate Reproductive Activities with Metabolism.Semin Reprod Med. 2019 May;37(3):131-140. doi: 10.1055/s-0039-3400251. Epub 2019 Dec 23. Semin Reprod Med. 2019. PMID: 31869841 Free PMC article. Review.
-
Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia.Neuropsychopharmacology. 2010 Nov;35(12):2462-78. doi: 10.1038/npp.2010.129. Epub 2010 Aug 25. Neuropsychopharmacology. 2010. PMID: 20736993 Free PMC article.
-
Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression.BMC Biol. 2007 Apr 26;5:16. doi: 10.1186/1741-7007-5-16. BMC Biol. 2007. PMID: 17462082 Free PMC article.
-
Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH.Psychopharmacology (Berl). 2008 Sep;200(1):105-15. doi: 10.1007/s00213-008-1161-4. Epub 2008 Jul 5. Psychopharmacology (Berl). 2008. PMID: 18604523 Free PMC article.
-
Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity.Neuropsychopharmacology. 2015 Aug;40(9):2103-12. doi: 10.1038/npp.2015.67. Epub 2015 Mar 10. Neuropsychopharmacology. 2015. PMID: 25754760 Free PMC article.
References
-
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. - PubMed
-
- Arjune D, Bodnar RJ. Suppression of nocturnal, palatable and glucoprivic intake in rats by the kappa opioid antagonist, norbinaltorphamine. Brain Res. 1990;534:313–316. - PubMed
-
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res. 1992;589:291–301. - PubMed
-
- Belluzzi JD, Stein L. Enkephaline may mediate euphoria and drive-reduction reward. Nature. 1977;266:556–558. - PubMed
-
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

