Improved quantitative structure-activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems
- PMID: 17166721
- PMCID: PMC2013303
- DOI: 10.1016/j.bmc.2006.11.037
Improved quantitative structure-activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems
Abstract
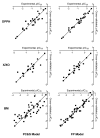
Quantitative structure-activity relationship (QSAR) models are useful in understanding how chemical structure relates to the biological activity of natural and synthetic chemicals and for design of newer and better therapeutics. In the present study, 46 flavonoids and related polyphenols were evaluated for direct/indirect antioxidant activity in three different assay systems of increasing complexity (chemical, enzymatic, and intact phagocytes). Based on these data, two different QSAR models were developed using (i) physicochemical and structural (PC&S) descriptors to generate multiparameter partial least squares (PLS) regression equations derived from optimized molecular structures of the tested compounds and (ii) a partial 3D comparison of the 46 compounds with local fingerprints obtained from fragments of the molecules by the frontal polygon (FP) method. We obtained much higher QSAR correlation coefficients (r) for flavonoid end-point antioxidant activity in all three assay systems using the FP method (0.966, 0.948, and 0.965 for datasets evaluated in the biochemical, enzymatic, and whole cell assay systems, respectively). Furthermore, high leave-one-out cross-validation coefficients (q2) of 0.907, 0.821, and 0.897 for these datasets, respectively, indicated enhanced predictive ability and robustness of the model. Using the FP method, structural fragments (submolecules) responsible for the end-point antioxidant activity in the three assay systems were also identified. To our knowledge, this is the first QSAR model derived for description of flavonoid direct/indirect antioxidant effects in a cellular system, and this model could form the basis for further drug development of flavonoid-like antioxidant compounds with therapeutic potential.
Figures


References
-
- Rice-Evans C. Curr Med Chem. 2001;8:797. - PubMed
-
- Ross JA, Kasum CM. Annu Rev Nutr. 2002;22:19. - PubMed
-
- Williams RJ, Spencer JP, Rice-Evans C. Free Radic Biol Med. 2004;36:838. - PubMed
-
- Heim KE, Tagliaferro AR, Bobilya DJ. J Nutr Biochem. 2002;13:572. - PubMed
-
- Wolin MS, Ahmad M, Gupte SA. Kidney Int. 2005;67:1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

