The cellular protein P58IPK regulates influenza virus mRNA translation and replication through a PKR-mediated mechanism
- PMID: 17166899
- PMCID: PMC1865913
- DOI: 10.1128/JVI.02151-06
The cellular protein P58IPK regulates influenza virus mRNA translation and replication through a PKR-mediated mechanism
Abstract
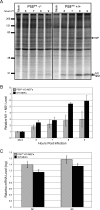
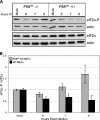
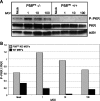
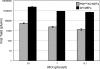
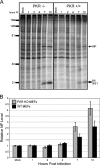
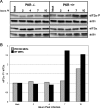
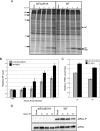
We previously hypothesized that efficient translation of influenza virus mRNA requires the recruitment of P58(IPK), the cellular inhibitor of PKR, an interferon-induced kinase that targets the eukaryotic translation initiation factor eIF2alpha. P58(IPK) also inhibits PERK, an eIF2alpha kinase that is localized in the endoplasmic reticulum (ER) and induced during ER stress. The ability of P58(IPK) to interact with and inhibit multiple eIF2alpha kinases suggests it is a critical regulator of both cellular and viral mRNA translation. In this study, we sought to definitively define the role of P58(IPK) during viral infection of mammalian cells. Using mouse embryo fibroblasts from P58(IPK-/-) mice, we demonstrated that the absence of P58(IPK) led to an increase in eIF2alpha phosphorylation and decreased influenza virus mRNA translation. The absence of P58(IPK) also resulted in decreased vesicular stomatitis virus replication but enhanced reovirus yields. In cells lacking the P58(IPK) target, PKR, the trends were reversed-eIF2alpha phosphorylation was decreased, and influenza virus mRNA translation was increased. Although P58(IPK) also inhibits PERK, the presence or absence of this kinase had little effect on influenza virus mRNA translation, despite reduced levels of eIF2alpha phosphorylation in cells lacking PERK. Finally, we showed that influenza virus protein synthesis and viral mRNA levels decrease in cells that express a constitutively active, nonphosphorylatable eIF2alpha. Taken together, our results support a model in which P58(IPK) regulates influenza virus mRNA translation and infection through a PKR-mediated mechanism which is independent of PERK.
Figures
Similar articles
-
Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK.Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):15920-5. doi: 10.1073/pnas.252341799. Epub 2002 Nov 22. Proc Natl Acad Sci U S A. 2002. PMID: 12446838 Free PMC article.
-
Influenza A virus nucleoprotein exploits Hsp40 to inhibit PKR activation.PLoS One. 2011;6(6):e20215. doi: 10.1371/journal.pone.0020215. Epub 2011 Jun 15. PLoS One. 2011. PMID: 21698289 Free PMC article.
-
Inactivation of the PKR protein kinase and stimulation of mRNA translation by the cellular co-chaperone P58(IPK) does not require J domain function.Biochemistry. 2002 Apr 16;41(15):4938-45. doi: 10.1021/bi0121499. Biochemistry. 2002. PMID: 11939789
-
Control of mRNA splicing by noncoding intragenic RNA elements that evoke a cellular stress response.Int J Biochem Cell Biol. 2018 Dec;105:20-23. doi: 10.1016/j.biocel.2018.09.021. Epub 2018 Sep 30. Int J Biochem Cell Biol. 2018. PMID: 30282053 Review.
-
The role of host eIF2α in viral infection.Virol J. 2020 Jul 23;17(1):112. doi: 10.1186/s12985-020-01362-6. Virol J. 2020. PMID: 32703221 Free PMC article. Review.
Cited by
-
Tinkering with translation: protein synthesis in virus-infected cells.Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a012351. doi: 10.1101/cshperspect.a012351. Cold Spring Harb Perspect Biol. 2013. PMID: 23209131 Free PMC article. Review.
-
Induction of Oxidative DNA Damage in Bovine Herpesvirus 1 Infected Bovine Kidney Cells (MDBK Cells) and Human Tumor Cells (A549 Cells and U2OS Cells).Viruses. 2018 Jul 26;10(8):393. doi: 10.3390/v10080393. Viruses. 2018. PMID: 30049996 Free PMC article.
-
The Role of Nucleic Acid Sensing in Controlling Microbial and Autoimmune Disorders.Int Rev Cell Mol Biol. 2019;345:35-136. doi: 10.1016/bs.ircmb.2018.08.002. Epub 2018 Sep 25. Int Rev Cell Mol Biol. 2019. PMID: 30904196 Free PMC article. Review.
-
The NS1 protein of influenza A virus suppresses interferon-regulated activation of antigen-presentation and immune-proteasome pathways.J Gen Virol. 2011 Sep;92(Pt 9):2093-2104. doi: 10.1099/vir.0.032060-0. Epub 2011 May 18. J Gen Virol. 2011. PMID: 21593271 Free PMC article.
-
Tipping the balance: antagonism of PKR kinase and ADAR1 deaminase functions by virus gene products.J Interferon Cytokine Res. 2009 Sep;29(9):477-87. doi: 10.1089/jir.2009.0065. J Interferon Cytokine Res. 2009. PMID: 19715457 Free PMC article. Review.
References
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. - PubMed
-
- Baltzis, D., L. K. Qu, S. Papadopoulou, J. D. Blais, J. C. Bell, N. Sonenberg, and A. E. Koromilas. 2004. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 78:12747-12761. - PMC - PubMed
-
- Bi, M., C. Naczki, M. Koritzinsky, D. Fels, J. Blias, N. Hu, H. P. Harding, I. Novoa, M. Varia, J. Raleigh, D. Scheuner, R. J. Kaufman, J. Bell, D. Ron, B. G. Wouters, and C. Koumenis. 2006. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24:3470-3481. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

