HLA-A2-restricted protection against lethal lymphocytic choriomeningitis
- PMID: 17166907
- PMCID: PMC1865925
- DOI: 10.1128/JVI.02063-06
HLA-A2-restricted protection against lethal lymphocytic choriomeningitis
Abstract
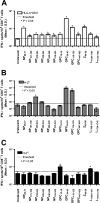
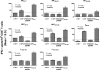
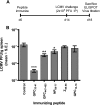
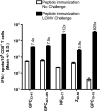
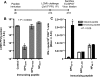
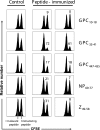
The consequences of human lymphocytic choriomeningitis virus (LCMV) infection can be severe, including aseptic meningitis in immunocompetent individuals, hydrocephalus or chorioretinitis in fetal infection, or a highly lethal outcome in immunosuppressed individuals. In murine models of LCMV infection, CD8(+) T cells play a primary role in providing protective immunity, and there is evidence that cellular immunity may also be important in related arenavirus infections in humans. For this reason, we sought to identify HLA-A2 supertype-restricted epitopes from the LCMV proteome and evaluate them as vaccine determinants in HLA transgenic mice. We identified four HLA-A*0201-restricted peptides-nucleoprotein NP(69-77), glycoprotein precursor GPC(10-18), GPC(447-455), and zinc-binding protein Z(49-58)-that displayed high-affinity binding (< or =275 nM) to HLA-A*0201, induced CD8(+) T-cell responses of high functional avidity in HLA-A*0201 transgenic mice, and were naturally processed from native LCMV antigens in HLA-restricted human antigen presenting cells. One of the epitopes (GPC(447-455)), after peptide immunization of HLA-A*0201 mice, induced CD8(+) T cells capable of killing peptide-pulsed HLA-A*0201-restricted target cells in vivo and protected mice against lethal intracranial challenge with LCMV.
Figures

Similar articles
-
Coverage of related pathogenic species by multivalent and cross-protective vaccine design: arenaviruses as a model system.Microbiol Mol Biol Rev. 2010 Jun;74(2):157-70. doi: 10.1128/MMBR.00045-09. Microbiol Mol Biol Rev. 2010. PMID: 20508245 Free PMC article. Review.
-
A multivalent vaccination strategy for the prevention of Old World arenavirus infection in humans.J Virol. 2010 Oct;84(19):9947-56. doi: 10.1128/JVI.00672-10. Epub 2010 Jul 28. J Virol. 2010. PMID: 20668086 Free PMC article.
-
Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein.Eur J Immunol. 1989 Mar;19(3):417-24. doi: 10.1002/eji.1830190302. Eur J Immunol. 1989. PMID: 2468501
-
Replication-incompetent rabies virus vector harboring glycoprotein gene of lymphocytic choriomeningitis virus (LCMV) protects mice from LCMV challenge.PLoS Negl Trop Dis. 2018 Apr 16;12(4):e0006398. doi: 10.1371/journal.pntd.0006398. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29659579 Free PMC article.
-
Direct ex vivo kinetic and phenotypic analyses of CD8(+) T-cell responses induced by DNA immunization.J Virol. 2000 Sep;74(18):8286-91. doi: 10.1128/jvi.74.18.8286-8291.2000. J Virol. 2000. PMID: 10954526 Free PMC article.
Cited by
-
A multivalent and cross-protective vaccine strategy against arenaviruses associated with human disease.PLoS Pathog. 2009 Dec;5(12):e1000695. doi: 10.1371/journal.ppat.1000695. Epub 2009 Dec 18. PLoS Pathog. 2009. PMID: 20019801 Free PMC article.
-
Strand-Specific Quantitative Reverse Transcription-Polymerase Chain Reaction Assay for Measurement of Arenavirus Genomic and Antigenomic RNAs.PLoS One. 2015 May 15;10(5):e0120043. doi: 10.1371/journal.pone.0120043. eCollection 2015. PLoS One. 2015. PMID: 25978311 Free PMC article.
-
Coverage of related pathogenic species by multivalent and cross-protective vaccine design: arenaviruses as a model system.Microbiol Mol Biol Rev. 2010 Jun;74(2):157-70. doi: 10.1128/MMBR.00045-09. Microbiol Mol Biol Rev. 2010. PMID: 20508245 Free PMC article. Review.
-
Evolutionarily conserved protein sequences of influenza a viruses, avian and human, as vaccine targets.PLoS One. 2007 Nov 21;2(11):e1190. doi: 10.1371/journal.pone.0001190. PLoS One. 2007. PMID: 18030326 Free PMC article.
-
HLA class I supertypes: a revised and updated classification.BMC Immunol. 2008 Jan 22;9:1. doi: 10.1186/1471-2172-9-1. BMC Immunol. 2008. PMID: 18211710 Free PMC article.
References
-
- Adair, C. V., R. L. Gauld, and J. E. Smadel. 1953. Aesptic meningitis, a disease of diverse etiology: clinical and etiologic studies on 854 cases. Ann. Int. Med. 39:675-704. - PubMed
-
- Barton, L. L., S. C. Budd, W. S. Morfitt, C. J. Peters, T. G. Ksiazek, R. F. Schindler, and M. T. Yoshino. 1993. Congenital lymphocytic choriomeningitis virus infection in twins. Pediatr. Infect. Dis. J. 12:942-946. - PubMed
-
- Barton, L. L., M. B. Mets, and C. L. Beauchamp. 2002. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 187:1715-1716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

