Pseudotyped single-cycle simian immunodeficiency viruses expressing gamma interferon augment T-cell priming responses in vitro
- PMID: 17166912
- PMCID: PMC1865962
- DOI: 10.1128/JVI.01879-06
Pseudotyped single-cycle simian immunodeficiency viruses expressing gamma interferon augment T-cell priming responses in vitro
Abstract
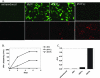
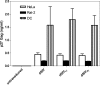
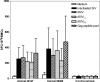
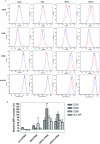
To increase the safety and efficacy of human immunodeficiency virus vaccines, several groups have conducted studies using the macaque model with single-cycle replicating simian immunodeficiency viruses (SIVs). However, these constructs had poor or diminished efficacy compared to live attenuated vaccines. We previously showed that immunization of macaques with live attenuated SIV with a deletion in the nef gene and expressing gamma interferon (IFN-gamma) results in significantly enhanced safety and efficacy. To further enhance safety, we constructed and characterized single-cycle SIVs, pseudotyped with the glycoprotein of vesicular stomatitis virus, expressing different levels of macaque IFN-gamma. Expression of IFN-gamma did not alter the infectivity or antigenicity of pseudotyped SIV. The transduction of dendritic cells (DCs) by IFN-gamma-expressing particles resulted in the up-regulation of costimulatory and major histocompatibility complex molecules. Furthermore, T cells primed with DCs transduced by SIV particles expressing high levels of IFN-gamma and then stimulated with SIV induced significantly higher numbers of spot-forming cells in an enzyme-linked immunospot assay than did T cells primed with DCs transduced with SIV particles lacking the cytokine. In conclusion, we demonstrated that the transduction of DCs in vitro with pseudotyped single-cycle SIVs expressing IFN-gamma increased DC activation and augmented T-cell priming activity.
Figures

Similar articles
-
Lower levels of gamma interferon expressed by a pseudotyped single-cycle simian immunodeficiency virus enhance immunogenicity in rats.J Virol. 2009 Feb;83(4):1592-601. doi: 10.1128/JVI.01446-08. Epub 2008 Dec 10. J Virol. 2009. PMID: 19073726 Free PMC article.
-
Evaluation of a self-inactivating lentiviral vector expressing simian immunodeficiency virus gag for induction of specific immune responses in vitro and in vivo.Viral Immunol. 2006 Winter;19(4):690-701. doi: 10.1089/vim.2006.19.690. Viral Immunol. 2006. PMID: 17201664
-
Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239.J Virol. 2005 Jun;79(12):7707-20. doi: 10.1128/JVI.79.12.7707-7720.2005. J Virol. 2005. PMID: 15919923 Free PMC article.
-
The interaction of immunodeficiency viruses with dendritic cells.Curr Top Microbiol Immunol. 2003;276:1-30. doi: 10.1007/978-3-662-06508-2_1. Curr Top Microbiol Immunol. 2003. PMID: 12797441 Review.
-
Interferon-gamma: producer cells, activation stimuli, and molecular genetic regulation.Pharmacol Ther. 1990;45(1):137-51. doi: 10.1016/0163-7258(90)90012-q. Pharmacol Ther. 1990. PMID: 2105509 Review. No abstract available.
Cited by
-
Lower levels of gamma interferon expressed by a pseudotyped single-cycle simian immunodeficiency virus enhance immunogenicity in rats.J Virol. 2009 Feb;83(4):1592-601. doi: 10.1128/JVI.01446-08. Epub 2008 Dec 10. J Virol. 2009. PMID: 19073726 Free PMC article.
-
Incorporation of CD40 ligand into the envelope of pseudotyped single-cycle Simian immunodeficiency viruses enhances immunogenicity.J Virol. 2009 Feb;83(3):1216-27. doi: 10.1128/JVI.01870-08. Epub 2008 Nov 26. J Virol. 2009. PMID: 19036823 Free PMC article.
References
-
- Ahuja, S. S., M. R. Brown, T. A. Fleisher, S. K. Ahuja, and H. L. Malech. 1996. Autocrine activation of hemopoietic progenitor-derived myelo-monocytic cells by IFN-gamma gene transfer. J. Immunol. 156:4345-4353. - PubMed
-
- Anderson, K. P., E. H. Fennie, and T. Yilma. 1988. Enhancement of a secondary antibody response to vesicular stomatitis virus “G” protein by IFN-gamma treatment at primary immunization. J. Immunol. 140:3599-3604. - PubMed
-
- Andrews, D. M., C. E. Andoniou, A. A. Scalzo, S. L. van Dommelen, M. E. Wallace, M. J. Smyth, and M. A. Degli-Esposti. 2005. Cross-talk between dendritic cells and natural killer cells in viral infection. Mol. Immunol. 42:547-555. - PubMed
-
- Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

