Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin
- PMID: 17166913
- PMCID: PMC1865921
- DOI: 10.1128/JVI.02302-06
Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin
Abstract
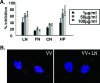
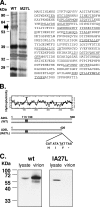
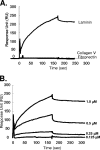
Vaccinia virus intracellular mature virus (IMV) binds to glycosaminoglycans (GAGs) on cells via three virion proteins, H3L, A27L, and D8L. In this study, we demonstrated that binding of IMV to BSC40 cells was competitively inhibited by soluble laminin but not by fibronectin or collagen V, suggesting that this cell surface extracellular matrix (ECM) protein may play a role in vaccinia virus entry. Moreover, IMV infection of GAG(-) sog9 cells was also inhibited by laminin, demonstrating that virion binding to laminin does not involve a prior interaction with GAGs. Furthermore, comparative envelope protein analyses of wild-type vaccinia virus strain Western Reserve, which binds to laminin, and of a mutant virus, IA27L, which does not, showed that the A26L open reading frame (ORF), encoding an envelope protein, was mutated in IA27L, resulting in A26L being absent from the IMV. Expression of the wild-type A26L ORF in IA27L resulted in laminin binding activity. Moreover, recombinant A26L protein bound to laminin in vitro with a high affinity, providing direct evidence that A26L is the laminin binding protein on IMV. In summary, these results reveal a novel role for the vaccinia viral envelope protein A26L in binding to the ECM protein laminin, an association that is proposed to facilitate IMV entry.
Figures




References
-
- Adamson, A. W. 1967. Physical chemistry of surfaces, p. 397-401. Interscience, New York, NY.
-
- Armstrong, J. A., D. H. Metz, and M. R. Young. 1973. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 21:533-537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

