Progressive deafness and altered cochlear innervation in knock-out mice lacking prosaposin
- PMID: 17167097
- PMCID: PMC6674959
- DOI: 10.1523/JNEUROSCI.3746-06.2006
Progressive deafness and altered cochlear innervation in knock-out mice lacking prosaposin
Abstract
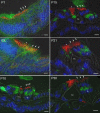
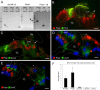
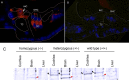
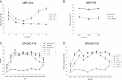
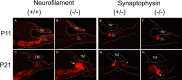
After a yeast two-hybrid screen identified prosaposin as a potential interacting protein with the nicotinic acetylcholine receptor (nAChR) subunit alpha10, studies were performed to characterize prosaposin in the normal rodent inner ear. Prosaposin demonstrates diffuse organ of Corti expression at birth, with gradual localization to the inner hair cells (IHCs) and its supporting cells, inner pillar cells, and synaptic region of the outer hair cells (OHCs) and Deiters' cells (DCs) by postnatal day 21 (P21). Microdissected OHC and DC quantitative reverse transcriptase-PCR and immunohistology localizes prosaposin mRNA to DCs and OHCs, and protein predominantly to the apex of the DCs. Subsequent studies in a prosaposin knock-out (KO) (-/-) mouse showed intact but slightly reduced hearing through P19, but deafness by P25 and reduced distortion product otoacoustic emissions from P15 onward. Beginning at P12, the prosaposin KO mice showed histologic organ of Corti changes including cellular hypertrophy in the region of the IHC and greater epithelial ridge, a loss of OHCs from cochlear apex, and vacuolization of OHCs. Immunofluorescence revealed exuberant overgrowth of auditory afferent neurites in the region of the IHCs and proliferation of auditory efferent neurites in the region of the tunnel of Corti. IHC recordings from these KO mice showed normal I-V curves and responses to applied acetylcholine. Together, these results suggest that prosaposin helps maintain normal innervation patterns to the organ of Corti. Furthermore, prosaposin's overlapping developmental expression pattern and binding capacity toward the nAChR alpha10 suggest that alpha10 may also play a role in this function.
Figures





References
-
- Baker ER, Zwart R, Sher E, Millar NS. Pharmacological properties of α9α10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol Pharmacol. 2004;65:453–460. - PubMed
-
- Berglund AM, Ryugo DK. Neurofilament antibodies and spiral ganglion neurons of the mammalian cochlea. J Comp Neurol. 1991;306:393–408. - PubMed
-
- Campana WM, Eskeland N, Calcutt NA, Misasi R, Myers RR, O'Brien JS. Prosaptide prevents paclitaxel neurotoxicity. Neurotoxicology. 1998;19:237–244. - PubMed
-
- Campana WM, Mohiuddin L, Misasi R, O'Brien JS, Calcutt NA. Prosaposin-derived peptides enhanced sprouting of sensory neurons in vitro and induced sprouting at motor endplates in vivo. J Peripher Nerv Syst. 2000;5:126–130. - PubMed
-
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
