N-terminal and C-terminal cytosine deaminase domain of APOBEC3G inhibit hepatitis B virus replication
- PMID: 17167839
- PMCID: PMC4087596
- DOI: 10.3748/wjg.v12.i46.7488
N-terminal and C-terminal cytosine deaminase domain of APOBEC3G inhibit hepatitis B virus replication
Abstract
Aim: To investigate the effect of human apolipoprotein B mRNA-editing enzyme catalytic-polypeptide 3G (APOBEC3G) and its N-terminal or C-terminal cytosine deaminase domain-mediated antiviral activity against hepatitis B virus (HBV) in vitro and in vivo.
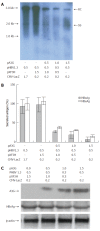
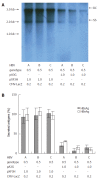
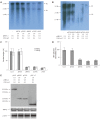
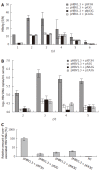
Methods: The mammalian hepatoma cells HepG2 and HuH7 were cotransfected with APOBEC3G and its N-terminal or C-terminal cytosine deaminase domain expression vector and 1.3-fold-overlength HBV DNA as well as the linear monomeric HBV of genotype B and C. For in vivo study, an HBV vector-based mouse model was used in which APOBEC3G and its N-terminal or C-terminal cytosine deaminase domain expression vectors were co-delivered with 1.3-fold-overlength HBV DNA via high-volume tail vein injection. Levels of hepatitis B virus surface antigen (HBsAg) and hepatitis B virus e antigen (HBeAg) in the media of the transfected cells and in the sera of mice were determined by ELISA. The expression of hepatitis B virus core antigen (HBcAg) in the transfected cells was determined by Western blot analysis. Core-associated HBV DNA was examined by Southern blot analysis. Levels of HBV DNA in the sera of mice as well as HBV core-associated RNA in the liver of mice were determined by quantitative PCR and quantitative RT-PCR analysis, respectively.
Results: Human APOBEC3G exerted an anti-HBV activity in a dose-dependent manner in HepG2 cells, and comparable suppressive effects were observed on genotype B and C as that of genotype A. Interestingly, the N-terminal or C-terminal cytosine deaminase domain alone could also inhibit HBV replication in HepG2 cells as well as Huh7 cells. Consistent with in vitro results, the levels of HBsAg in the sera of mice were dramatically decreased, with more than 50 times decrease in the levels of serum HBV DNA and core-associated RNA in the liver of mice treated with APOBEC3G and its N-terminal or C-terminal cytosine deaminase domain as compared to the controls.
Conclusion: Our findings provide probably the first evidence showing that APOBEC3G and its N-terminal or C-terminal cytosine deaminase domain could suppress HBV replication in vitro and in vivo.
Figures
Similar articles
-
Associations between activation-induced cytidine deaminase/apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like cytidine deaminase expression, hepatitis B virus (HBV) replication and HBV-associated liver disease (Review).Mol Med Rep. 2015 Nov;12(5):6405-14. doi: 10.3892/mmr.2015.4312. Epub 2015 Sep 10. Mol Med Rep. 2015. PMID: 26398702 Free PMC article. Review.
-
Inhibition of hepatitis B virus replication by APOBEC3G in vitro and in vivo.World J Gastroenterol. 2006 Jul 28;12(28):4492-7. doi: 10.3748/wjg.v12.i28.4492. World J Gastroenterol. 2006. PMID: 16874860 Free PMC article.
-
[Inhibition of hepatitis B and duck hepatitis B virus replication by APOBEC3G].Zhonghua Gan Zang Bing Za Zhi. 2006 Oct;14(10):738-41. Zhonghua Gan Zang Bing Za Zhi. 2006. PMID: 17064466 Chinese.
-
Dual effect of APOBEC3G on Hepatitis B virus.J Gen Virol. 2007 Feb;88(Pt 2):432-440. doi: 10.1099/vir.0.82319-0. J Gen Virol. 2007. PMID: 17251560
-
APOBEC deaminases as cellular antiviral factors: a novel natural host defense mechanism.Med Sci Monit. 2006 May;12(5):RA92-8. Med Sci Monit. 2006. PMID: 16641889 Review.
Cited by
-
The Intricate Interplay between APOBEC3 Proteins and DNA Tumour Viruses.Pathogens. 2024 Feb 20;13(3):187. doi: 10.3390/pathogens13030187. Pathogens. 2024. PMID: 38535531 Free PMC article. Review.
-
Associations between activation-induced cytidine deaminase/apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like cytidine deaminase expression, hepatitis B virus (HBV) replication and HBV-associated liver disease (Review).Mol Med Rep. 2015 Nov;12(5):6405-14. doi: 10.3892/mmr.2015.4312. Epub 2015 Sep 10. Mol Med Rep. 2015. PMID: 26398702 Free PMC article. Review.
-
Roles of APOBEC3 in hepatitis B virus (HBV) infection and hepatocarcinogenesis.Bioengineered. 2021 Dec;12(1):2074-2086. doi: 10.1080/21655979.2021.1931640. Bioengineered. 2021. PMID: 34043485 Free PMC article. Review.
-
HIV-1 Vif, APOBEC, and intrinsic immunity.Retrovirology. 2008 Jun 24;5:51. doi: 10.1186/1742-4690-5-51. Retrovirology. 2008. PMID: 18577210 Free PMC article. Review.
-
Genetic editing of HBV DNA by monodomain human APOBEC3 cytidine deaminases and the recombinant nature of APOBEC3G.PLoS One. 2009;4(1):e4277. doi: 10.1371/journal.pone.0004277. Epub 2009 Jan 26. PLoS One. 2009. PMID: 19169351 Free PMC article.
References
-
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. - PubMed
-
- Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. - PubMed
-
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

