Hepatotoxicity induced by cyproterone acetate: a report of three cases
- PMID: 17167851
- PMCID: PMC4087608
- DOI: 10.3748/wjg.v12.i46.7551
Hepatotoxicity induced by cyproterone acetate: a report of three cases
Abstract
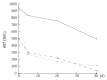
Cyproterone acetate (CPA) is a steroidal synthetic progestagen and anti-androgenic compound widely administered in prostate cancer which has been evidentially correlated with a severe hepatotoxic potency. Three male patients aged 78-83 years are presented, in whom severe hepatotoxic reactions emerged after CPA administration. Patients were treated with CPA at the doses of 200-300 mg/d for malignant prostate disease for 3-12 mo prior to the acute manifestation of the hepatic disease. Clinical features compatible with mixed hepatocellular and cholestatic liver disease including jaundice, white stools and dark urine, manifested in all three cases whereas encephalopathy and ascites were present in two of the patients. Other primary causes of hepatotoxicity (alcohol consumption and viral hepatitis) were also verified in two cases, and in those patients biopsy findings revealed the presence of cirrhotic lesions in liver parenchyma. Discontinuation of the therapeutic agent led to the amelioration of the clinical profile in all the patients whereas a patient died 40 d after hospital admission due to sepsis, despite acute liver disease improvement. The current article highlights the hepatotoxic potency of a widely administered therapeutic agent and illustrates the importance of clinical surveillance especially in patients with previous hepatic diseases. Three relevant cases are reported and a review of the published literature is made.
Figures


References
-
- Lévesque H, Trivalle C, Manchon ND, Vinel JP, Moore N, Hémet J, Courtois H, Bercoff E, Bourreille J. Fulminant hepatitis due to cyproterone acetate. Lancet. 1989;1:215–216. - PubMed
-
- Murphy BJ, Collins BJ. Severe hepatitis and liver failure induced by cyproterone acetate. Aust N Z J Med. 1996;26:724. - PubMed
-
- Drakos PE, Gez E, Catane R. Hepatitis due to cyproterone acetate. Eur J Cancer. 1992;28A:1931–1932. - PubMed
-
- Roila F, Crinò L, Carloni G, Natalini G. Cyproterone acetate: hepatotoxicity and prostatic cancer treatment. Ann Oncol. 1993;4:701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

