Rings, radicals, and regeneration: the early years of a bioorganic laboratory
- PMID: 17168571
- PMCID: PMC2519235
- DOI: 10.1021/jo0614240
Rings, radicals, and regeneration: the early years of a bioorganic laboratory
Abstract
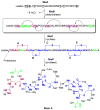
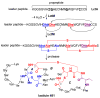
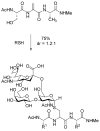
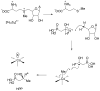
This Perspective provides an overview of the progress in two of the original programs in my research group focused on the biosynthesis of the antibiotics nisin, lacticin 481, fosfomycin, and bialaphos. The path from start-up funds to tenure and beyond offers insights into the opportunities realized and missed along the road.
Figures
















Similar articles
-
First evidence of production of the lantibiotic nisin P.Sci Rep. 2020 Feb 28;10(1):3738. doi: 10.1038/s41598-020-60623-0. Sci Rep. 2020. PMID: 32111904 Free PMC article.
-
Mechanistic dissection of the enzyme complexes involved in biosynthesis of lacticin 3147 and nisin.Appl Environ Microbiol. 2008 Nov;74(21):6591-7. doi: 10.1128/AEM.01334-08. Epub 2008 Sep 12. Appl Environ Microbiol. 2008. PMID: 18791001 Free PMC article.
-
Nisin and lacticin 481 coproduction by Lactococcus lactis strains isolated from raw ewes' milk.J Dairy Sci. 2009 Oct;92(10):4805-11. doi: 10.3168/jds.2009-2237. J Dairy Sci. 2009. PMID: 19762795
-
Bacteriocins: safe, natural antimicrobials for food preservation.Int J Food Microbiol. 2001 Dec 4;71(1):1-20. doi: 10.1016/s0168-1605(01)00560-8. Int J Food Microbiol. 2001. PMID: 11764886 Review.
-
Bioengineering of the model lantibiotic nisin.Bioengineered. 2015;6(4):187-92. doi: 10.1080/21655979.2015.1049781. Epub 2015 May 13. Bioengineered. 2015. PMID: 25970137 Free PMC article. Review.
Cited by
-
Different biosynthetic pathways to fosfomycin in Pseudomonas syringae and Streptomyces species.Antimicrob Agents Chemother. 2012 Aug;56(8):4175-83. doi: 10.1128/AAC.06478-11. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615277 Free PMC article.
-
Significant increase of oxidase activity through the genetic incorporation of a tyrosine-histidine cross-link in a myoglobin model of heme-copper oxidase.Angew Chem Int Ed Engl. 2012 Apr 27;51(18):4312-6. doi: 10.1002/anie.201108756. Epub 2012 Mar 12. Angew Chem Int Ed Engl. 2012. PMID: 22411709 Free PMC article.
-
Vitamin B12 in the spotlight again.Curr Opin Chem Biol. 2017 Apr;37:63-70. doi: 10.1016/j.cbpa.2017.01.013. Epub 2017 Feb 3. Curr Opin Chem Biol. 2017. PMID: 28167430 Free PMC article. Review.
-
Stereochemical and Mechanistic Investigation of the Reaction Catalyzed by Fom3 from Streptomyces fradiae, a Cobalamin-Dependent Radical S-Adenosylmethionine Methylase.Biochemistry. 2018 Aug 21;57(33):4972-4984. doi: 10.1021/acs.biochem.8b00693. Epub 2018 Aug 9. Biochemistry. 2018. PMID: 30036047 Free PMC article.
-
Radical S-adenosylmethionine enzymes.Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available.
References
-
- Li YM, Milne JC, Madison LL, Kolter R, Walsh CT. Science. 1996;274:1188–93. - PubMed
-
- Kuzuyama T, Hidaka T, Kamigiri K, Imai S, Seto H. J Antibiot. 1992;45:1812–4. - PubMed
- Kamigiri K, Hidaka T, Imai S, Murakami T, Seto H. J Antibiot. 1992;45:781–7. - PubMed
- Hidaka T, Goda M, Kuzuyama T, Takei N, Kidaka M, Seto H. Mol Gen Genet. 1995;249:274–280. - PubMed
- Hidaka T, Hidaka M, Kuzuyama T, Seto H. Gene. 1995;158:149–150. - PubMed
- Seto H, Kuzuyama T. Nat Prod Rep. 1999;16:589–596. - PubMed
- Seto H, Hidaka T, Kuzuyama T, Shibahara S, Usui T, Sakanaka O, Imai S. J Antibiot. 1991;44:1286–8. - PubMed
-
- Hammerschmidt F. Angew Chem Int Ed Engl. 1994;33:341–342.
- Hammerschmidt F, Kählig H. J Org Chem. 1991;56:2364–2370.
-
- Banerjee R, editor. The Chemistry and Biochemistry of B12. Wiley; New York: 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

