The use of genotyping in antimalarial clinical trials: a systematic review of published studies from 1995-2005
- PMID: 17169157
- PMCID: PMC1716173
- DOI: 10.1186/1475-2875-5-122
The use of genotyping in antimalarial clinical trials: a systematic review of published studies from 1995-2005
Abstract
Background: The use of genotyping to distinguish recrudescent from new infections is currently recommended for all clinical antimalarial efficacy trials by the World Health Organization. However, genotyping-adjusted drug efficacy estimates may vary between trials due to the use of different genotyping methods and to the different settings in which these methods are applied.
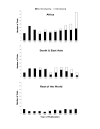
Methods: A systematic review of all clinical antimalarial efficacy trials published from 1995-2005 was performed to characterize the use of genotyping, including the methods used and the effect of these methods on estimates of drug efficacy.
Results: In a multivariate analysis, the method of interpretation of genotyping results, the studied therapy, the location of the trial, and the duration of study follow-up all had statistically significant effects on the percent of genotyped outcomes classified as new infections.
Conclusion: Criteria for defining appropriate, standardized genotyping methods for use in different settings are needed to enable more accurate estimates of antimalarial drug efficacy and better comparison between trials. The advantages and disadvantages of different genotyping methods and their potential impact in various settings are discussed.
Figures
References
-
- World Malaria Report http://www.rbm.who.int/wmr2005/
-
- Susceptibility of Plasmodium falciparum to antimalarial drugs http://www.who.int/malaria/rbm/Attachment/20041108/SusceptibilityPlasmod...
-
- Guidelines for the Treatment of Malaria http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
-
- Farnert A, Arez AP, Babiker HA, Beck HP, Benito A, Bjorkman A, Bruce MC, Conway DJ, Day KP, Henning L, Mercereau-Puijalon O, Ranford-Cartwright LC, Rubio JM, Snounou G, Walliker D, Zwetyenga J, do Rosario VE. Genotyping of Plasmodium falciparum infections by PCR: a comparative multicentre study. Trans R Soc Trop Med Hyg. 2001;95:225–232. doi: 10.1016/S0035-9203(01)90175-0. - DOI - PubMed
-
- Slater M, Kiggundu M, Dokomajilar C, Kamya MR, Bakyaita N, Talisuna A, Rosenthal PJ, Dorsey G. Distinguishing recrudescences from new infections in antimalarial clinical trials: major impact of interpretation of genotyping results on estimates of drug efficacy. Am J Trop Med Hyg. 2005;73:256–262. - PubMed