Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: role of GILZ homo-dimerization and C-terminal domain
- PMID: 17169985
- PMCID: PMC1802615
- DOI: 10.1093/nar/gkl1080
Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: role of GILZ homo-dimerization and C-terminal domain
Expression of concern in
-
Expression of concern on 'glucocorticoid-induced leucine zipper (GILZ)/NF-κB interaction: role of GILZ homo-dimerization and C-terminal domain'.Nucleic Acids Res. 2022 Nov 11;50(20):12000. doi: 10.1093/nar/gkac1128. Nucleic Acids Res. 2022. PMID: 36370109 Free PMC article. No abstract available.
-
Editor's Note on 'Glucocorticoid-induced leucine zipper (GILZ)/NF-κB interaction: role of GILZ homo-dimerization and C-terminal domain'.Nucleic Acids Res. 2023 May 22;51(9):4699-4700. doi: 10.1093/nar/gkad287. Nucleic Acids Res. 2023. PMID: 37070212 Free PMC article. No abstract available.
Abstract
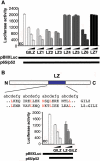
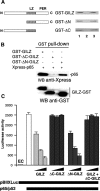
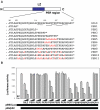
Glucocorticoid-induced leucine zipper (GILZ) is a 137 amino acid protein, rapidly induced by treatment with glucocorticoids (GC), characterized by a leucine zipper (LZ) domain (76-97 amino acids), an N-terminal domain (1-75 amino acids) and a C-terminal PER domain (98-137 amino acids) rich in proline and glutamic acid residues. We have previously shown that GILZ binds to and inhibits NF-kappaB activity. In the present study we used a number of mutants with the aim of defining the GILZ molecular domains responsible for GILZ/p65NF-kappaB interaction. Results, obtained by in vitro and in vivo co-immunoprecipitation (Co-IP) and by transcriptional activity experiments, indicate that GILZ homo-dimerization, through the LZ domain, as well as the C-terminal PER domain, particularly the 121-123 amino acids, are both necessary for GILZ interaction with NF-kappaB, inhibition of transcriptional activity and of IL-2 synthesis.
Figures


References
-
- Iwata M., Hanaoka S., Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur. J. Immunol. 1991;21:643–648. - PubMed
-
- Kwak L.W., Longo D.L. Lymphomas. Cancer Chemother. Biol. Response Modif. 1996;16:376–440. - PubMed
-
- Raff M.C. Social controls on cell survival and cell death. Nature. 1992;356:397–400. - PubMed
-
- Frankfurt O., Rosen S.T. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr. Opin. Oncol. 2004;16:553–563. - PubMed
-
- Barnes P.J., Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol. Sci. 1993;14:436–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

