Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis
- PMID: 17170135
- PMCID: PMC1702318
- DOI: 10.1073/pnas.0609140103
Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis
Abstract
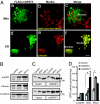
Acyl-CoA:glycerol-3-phosphate acyltransferase (GPAT) catalyzes the first step during de novo synthesis of triacylglycerol. It has been well recognized that mammals possess multiple enzymatically distinct proteins with GPAT activity. Although the mitochondrial-associated GPAT has been cloned and extensively characterized, the molecular identity of the endoplasmic reticulum (ER)-associated GPAT, which accounts for the majority of total GPAT activity in most tissues, has remained elusive. Here we report the identification of genes encoding human and mouse ER-associated GPAT (termed GPAT3). GPAT3 is a member of the acyltransferase family predominantly expressed in tissues characterized by active lipid metabolism, such as adipose tissue, small intestine, kidney, and heart. Ectopic expression of GPAT3 leads to a significant increase in N-ethylmaleimide-sensitive GPAT activity, whereas acyltransferase activity toward a variety of other lysophospholipids, as well as neutral lipid substrates, is not altered. Overexpression of GPAT3 in mammalian cells results in increased triacylglycerol, but not phospholipid, formation. GPAT3 is localized to the ER when overexpressed in COS-7 cells. GPAT3 mRNA is dramatically up-regulated during adipocyte differentiation, is reciprocally regulated in adipose tissue and liver of ob/ob mice, and is up-regulated in mice treated with a peroxisome proliferator-activated receptor gamma (PPARgamma) agonist. A substantial loss of GPAT activity in 3T3-L1 adipocytes was achieved by reducing GPAT3 mRNA levels through GPAT3-specific siRNA knockdown. These findings identify GPAT3 as a previously uncharacterized triacylglycerol biosynthetic enzyme. Similar to other lipogenic enzymes, GPAT3 may be useful as a target for the treatment of obesity.
Conflict of interest statement
Conflict of interest statement: H.F.L. owns publicly traded stock in Wyeth. The authors are employed by Wyeth and may also own publicly traded stock in Wyeth.
Figures
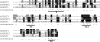




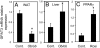
References
-
- Coleman RA, Lee DP. Prog Lipid Res. 2004;43:134–176. - PubMed
-
- Lehner R, Kuksis A. Prog Lipid Res. 1996;35:169–201. - PubMed
-
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Nat Genet. 2000;25:87–90. - PubMed
-
- Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr J Biol Chem. 2004;279:11767–11776. - PubMed
-
- Lewin TM, Schwerbrock NM, Lee DP, Coleman RA. J Biol Chem. 2004;279:13488–13495. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

