p21 delays tumor onset by preservation of chromosomal stability
- PMID: 17170138
- PMCID: PMC1702317
- DOI: 10.1073/pnas.0606343104
p21 delays tumor onset by preservation of chromosomal stability
Abstract
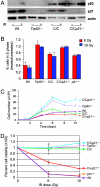
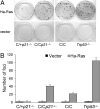
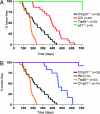
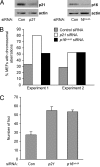
The p53 protein suppresses tumorigenesis by initiating cellular functions such as cell cycle arrest and apoptosis in response to DNA damage. A p53 mutant, p53R172P, which is deficient for apoptosis but retains a partial cell cycle arrest function, delays tumor onset in mice. Remarkably, lymphomas arising in Trp53(515C/515C) mice (encoding p53R172P) retain stable genomes. Given the dominant role of p21 in p53 cell cycle control, we crossed Trp53(515C/515C) mice onto a p21-null background to determine whether p21 was required for maintaining chromosomal stability and delaying tumor onset. Loss of p21 completely abolished the cell cycle arrest function of p53R172P and accelerated tumor onset in Trp53(515C/515C) mice. Cytogenetic examination of Trp53(515C/515C) p21(-/-) sarcomas and lymphomas revealed aneuploidy and chromosomal aberrations that were absent in Trp53(515C/515C) malignancies. Thus, p21 coupled p53-dependent checkpoint control and preservation of chromosomal stability, and cooperated with apoptosis in suppressing tumor onset in mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75:817–825. - PubMed
-
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. Cell. 1993;75:805–816. - PubMed
-
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Cell. 1995;82:675–684. - PubMed
-
- Waldman T, Kinzler KW, Vogelstein B. Cancer Res. 1995;55:5187–5190. - PubMed
-
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Nature. 1995;377:552–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

