Unfolded protein response in a Drosophila model for retinal degeneration
- PMID: 17170705
- PMCID: PMC1782370
- DOI: 10.1038/sj.emboj.7601477
Unfolded protein response in a Drosophila model for retinal degeneration
Abstract
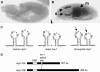
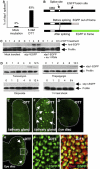
Stress in the endoplasmic reticulum (ER stress) and its cellular response, the unfolded protein response (UPR), are implicated in a wide variety of diseases, but its significance in many disorders remains to be validated in vivo. Here, we analyzed a branch of the UPR mediated by xbp1 in Drosophila to establish its role in neurodegenerative diseases. The Drosophila xbp1 mRNA undergoes ire-1-mediated unconventional splicing in response to ER stress, and this property was used to develop a specific UPR marker, xbp1-EGFP, in which EGFP is expressed in frame only after ER stress. xbp1-EGFP responds specifically to ER stress, but not to proteins that form cytoplasmic aggregates. The ire-1/xbp1 pathway regulates heat shock cognate protein 3 (hsc3), an ER chaperone. xbp1 splicing and hsc3 induction occur in the retina of ninaE(G69D)-/+, a Drosophila model for autosomal dominant retinitis pigmentosa (ADRP), and reduction of xbp1 gene dosage accelerates retinal degeneration of these animals. These results demonstrate the role of the UPR in the Drosophila ADRP model and open new opportunities for examining the UPR in other Drosophila disease models.
Figures




References
-
- Armknecht S, Boutros M, Kiger A, Nybakken K, Mathey-Prevot B, Perrimon N (2005) High-throughput RNA interference screens in Drosophila tissue culture cells. Methods Enzymol 392: 55–73 - PubMed
-
- Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ (2005) ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods 35: 395–416 - PubMed
-
- Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin–proteasome system by protein aggregation. Science 292: 1552–1555 - PubMed
-
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

