Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast
- PMID: 17170709
- PMCID: PMC1782361
- DOI: 10.1038/sj.emboj.7601466
Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast
Abstract
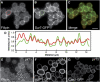
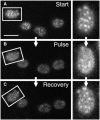
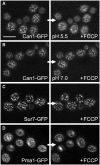
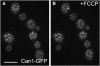
The plasma membrane potential is mainly considered as the driving force for ion and nutrient translocation. Using the yeast Saccharomyces cerevisiae as a model organism, we have discovered a novel role of the membrane potential in the organization of the plasma membrane. Within the yeast plasma membrane, two non-overlapping sub-compartments can be visualized. The first one, represented by a network-like structure, is occupied by the proton ATPase, Pma1, and the second one, forming 300-nm patches, houses a number of proton symporters (Can1, Fur4, Tat2 and HUP1) and Sur7, a component of the recently described eisosomes. Evidence is presented that sterols, the main lipid constituent of the plasma membrane, also accumulate within the patchy compartment. It is documented that this compartmentation is highly dependent on the energization of the membrane. Plasma membrane depolarization causes reversible dispersion of the H(+)-symporters, not however of the Sur7 protein. Mitochondrial mutants, affected in plasma membrane energization, show a significantly lower degree of membrane protein segregation. In accordance with these observations, depolarized membranes also considerably change their physical properties (detergent sensitivity).
Figures



References
-
- Aggeler R, Capaldi RA (1990) Yeast cytochrome c oxidase subunit VII is essential for assembly of an active enzyme. Cloning, sequencing, and characterization of the nuclear-encoded gene. J Biol Chem 265: 16389–16393 - PubMed
-
- Anderson RGW, Jacobson K (2002) A role of lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296: 1821–1825 - PubMed
-
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132 - PubMed
-
- Brown DA, London E (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14: 111–136 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

