Thermodynamics-based metabolic flux analysis
- PMID: 17172310
- PMCID: PMC1796839
- DOI: 10.1529/biophysj.106.093138
Thermodynamics-based metabolic flux analysis
Abstract
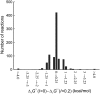
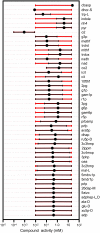
A new form of metabolic flux analysis (MFA) called thermodynamics-based metabolic flux analysis (TMFA) is introduced with the capability of generating thermodynamically feasible flux and metabolite activity profiles on a genome scale. TMFA involves the use of a set of linear thermodynamic constraints in addition to the mass balance constraints typically used in MFA. TMFA produces flux distributions that do not contain any thermodynamically infeasible reactions or pathways, and it provides information about the free energy change of reactions and the range of metabolite activities in addition to reaction fluxes. TMFA is applied to study the thermodynamically feasible ranges for the fluxes and the Gibbs free energy change, Delta(r)G', of the reactions and the activities of the metabolites in the genome-scale metabolic model of Escherichia coli developed by Palsson and co-workers. In the TMFA of the genome scale model, the metabolite activities and reaction Delta(r)G' are able to achieve a wide range of values at optimal growth. The reaction dihydroorotase is identified as a possible thermodynamic bottleneck in E. coli metabolism with a Delta(r)G' constrained close to zero while numerous reactions are identified throughout metabolism for which Delta(r)G' is always highly negative regardless of metabolite concentrations. As it has been proposed previously, these reactions with exclusively negative Delta(r)G' might be candidates for cell regulation, and we find that a significant number of these reactions appear to be the first steps in the linear portion of numerous biosynthesis pathways. The thermodynamically feasible ranges for the concentration ratios ATP/ADP, NAD(P)/NAD(P)H, and H(extracellular)(+)/H(intracellular)(+) are also determined and found to encompass the values observed experimentally in every case. Further, we find that the NAD/NADH and NADP/NADPH ratios maintained in the cell are close to the minimum feasible ratio and maximum feasible ratio, respectively.
Figures




References
-
- Alberty, R. A. 1998. Calculation of standard transformed formation properties of biochemical reactants and standard apparent reduction potentials of half reactions. Arch. Biochem. Biophys. 358:25–39. - PubMed
-
- Roels, J. A. 1983. Energetics and Kinetics in Biotechnology. Elsevier Biomedical Press, Amsterdam and New York.
-
- Westerhoff, H. V., and International Study Group of Biological Thermodynamics Control and Kinetics. 1994. Biothermokinetics. Intercept, Andover, Hampshire, England.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

