CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut
- PMID: 17172331
- PMCID: PMC1855769
- DOI: 10.1128/JB.01427-06
CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut
Abstract
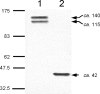
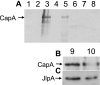
Two putative autotransporter proteins, CapA and CapB, were identified in silico from the genome sequence of Campylobacter jejuni NCTC11168. The genes encoding each protein contain homopolymeric tracts, suggestive of phase variation mediated by a slipped-strand mispairing mechanism; in each case the gene sequence contained frameshifts at these positions. The C-terminal two-thirds of the two genes, as well as a portion of the predicted signal peptides, were identical; the remaining N-terminal portions were gene specific. Both genes were cloned and expressed; recombinant polypeptides were purified and used to raise rabbit polyclonal monospecific antisera. Using immunoblotting, expression of the ca.116-kDa CapA protein was demonstrated for in vitro-grown cells of strain NCTC11168, for 4 out of 11 recent human fecal isolates, and for 2 out of 8 sequence-typed strains examined. Expression of CapB was not detected for any of the strains tested. Surface localization of CapA was demonstrated by subcellular fractionation and immunogold electron microscopy. Export of CapA was inhibited by globomycin, reinforcing the bioinformatic prediction that the protein is a lipoprotein. A capA insertion mutant had a significantly reduced capacity for association with and invasion of Caco-2 cells and failed to colonize and persist in chickens, indicating that CapA plays a role in host association and colonization by Campylobacter. In view of this demonstrated role, we propose that CapA stands for Campylobacter adhesion protein A.
Figures



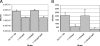
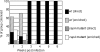
References
-
- Barenkamp, S. J., and J. W. St Geme III. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol. Microbiol. 19:1215-1223. - PubMed
-
- Baylis, C. L., S. MacPhee, K. W. Martin, T. J. Humphrey, and R. P. Betts. 2000. Comparison of three enrichment media for the isolation of Campylobacter spp. from foods. J. Appl. Microbiol. 89:884-891. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

