Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis
- PMID: 17172441
- PMCID: PMC1702530
- DOI: 10.1073/pnas.0609628104
Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis
Abstract
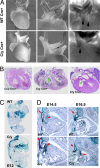
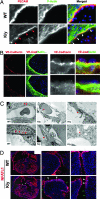
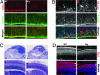
Mutations in the human Filamin A (FLNA) gene disrupt neuronal migration to the cerebral cortex and cause cardiovascular defects. Complete loss of Flna in mice results in embryonic lethality with severe cardiac structural defects involving ventricles, atria, and outflow tracts, as well as widespread aberrant vascular patterning. Despite these widespread developmental defects, migration and motility of many cell types does not appear to be affected. Instead, Flna-null embryos display abnormal epithelial and endothelial organization and aberrant adherens junctions in developing blood vessels, heart, brain, and other tissues. Essential roles for FLNA in intercellular junctions provide a mechanism for the diverse developmental defects seen in patients with FLNA mutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Feng Y, Walsh CA. Nat Cell Biol. 2004;6:1034–1038. - PubMed
-
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Nat Rev Mol Cell Biol. 2001;2:138–145. - PubMed
-
- Eksioglu YZ, Scheffer IE, Cardenas P, Knoll J, DiMario F, Ramsby G, Berg M, Kamuro K, Berkovic SF, Duyk GM, et al. Neuron. 1996;16:77–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

