Identification of specific chemoattractants and genetic complementation of a Borrelia burgdorferi chemotaxis mutant: flow cytometry-based capillary tube chemotaxis assay
- PMID: 17172459
- PMCID: PMC1828676
- DOI: 10.1128/AEM.01913-06
Identification of specific chemoattractants and genetic complementation of a Borrelia burgdorferi chemotaxis mutant: flow cytometry-based capillary tube chemotaxis assay
Abstract
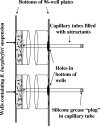
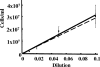
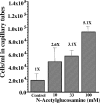
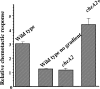
Measuring the chemotactic response of Borrelia burgdorferi, the bacterial species that causes Lyme disease, is relatively more difficult than measuring that of other bacteria. Because these spirochetes have long generation times, enumerating cells that swim up a capillary tube containing an attractant by using colony counts is impractical. Furthermore, direct counts with a Petroff-Hausser chamber is problematic, as this method has a low throughput and necessitates a high cell density; the latter can lead to misinterpretation of results when assaying for specific attractants. Only rabbit serum and tick saliva have been reported to be chemoattractants for B. burgdorferi. These complex biological mixtures are limited in their utility for studying chemotaxis on a molecular level. Here we present a modified capillary tube chemotaxis assay for B. burgdorferi that enumerates cells by flow cytometry. Initial studies identified N-acetylglucosamine as a chemoattractant. The assay was then optimized with respect to cell concentration, incubation time, motility buffer composition, and growth phase. Besides N-acetylglucosamine, glucosamine, glucosamine dimers (chitosan), glutamate, and glucose also elicited significant chemoattractant responses, although the response obtained with glucose was weak and variable. Serine and glycine were nonchemotactic. To further validate and to exploit the use of this assay, a previously described nonchemotactic cheA2 mutant was shown to be nonchemotactic by this assay; it also regained the wild-type phenotype when complemented in trans. This is the first report that identifies specific chemical attractants for B. burgdorferi and the use of flow cytometry for spirochete enumeration. The method should also be useful for assaying chemotaxis for other slow-growing prokaryotic species and in specific environments in nature.
Figures


References
-
- Adler, J. 1973. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol. 74:77-91. - PubMed
-
- Baker, M. D., P. M. Wolanin, and J. B. Stock. 2006. Signal transduction in bacterial chemotaxis. Bioessays 28:9-22. - PubMed
-
- Bakker, R. G. 2006. Measuring chemotaxis in Borrelia burgdorferi, the Lyme disease spirochete. Ph.D. dissertation. West Virginia University, Morgantown.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

