Arsenic disruption of steroid receptor gene activation: Complex dose-response effects are shared by several steroid receptors
- PMID: 17173375
- PMCID: PMC2556599
- DOI: 10.1021/tx060122q
Arsenic disruption of steroid receptor gene activation: Complex dose-response effects are shared by several steroid receptors
Abstract
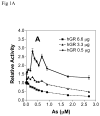
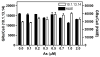
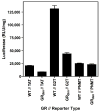
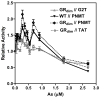
Chronic intake of arsenic (As) has been associated with increased risk of cancer, diabetes, developmental and reproductive problems, and cardiovascular disease. Recent studies suggest increased health risks with drinking water levels as low as 5-10 ppb. We previously reported that As disrupts glucocorticoid receptor (GR) mediated transcription in a very complex fashion. Low As levels (0.1-0.7 microM) stimulated transcription, whereas slightly higher levels (1-3 microM) were inhibitory. The DNA binding domain (DBD) was the minimal region of GR required for the response to As. Mutations in the DBD that alter the conformation of the dimerization domain (D-loop) to a DNA-bound GR conformation abolished the stimulatory effect and enhanced the inhibitory response to As. Here we report that receptors for progesterone (PR) and mineralocorticoids display a complex As response similar to that of the GR, suggesting a common mechanism for this effect. The complex response to As is not due to altered steroid or receptor levels. Moreover, a well-characterized GR dimerization mutant displayed a wild-type biphasic response to As for several divergent reporter genes, suggesting that dimerization is not critical for the response to As. Fluorescence polarization studies with purified PR and GR demonstrated that the specific PR/GR-DNA interaction is not altered in the presence of As. These results indicate that the numerous and diverse human health effects associated with As exposure may be mediated, at least in part, through its ability to simultaneously disrupt multiple hormone receptor systems.
Figures






References
-
- ATSDR. Toxicological Profile for Arsenic (Update) Agency for Toxic Substances and Disease Registry, SUDHHS, PHS; Washington D.C: 1999. - PubMed
-
- NRC. Arsenic in Drinking Water. National Research Council; Washington D.C.: 1999.
-
- Karagas MR, Stukel TA, Tosteson TD. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int J Hyg Environ Health. 2002;205:85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

