Phosphene induction by microstimulation of macaque V1
- PMID: 17173976
- PMCID: PMC1850969
- DOI: 10.1016/j.brainresrev.2006.11.001
Phosphene induction by microstimulation of macaque V1
Abstract
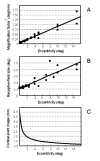
Non-human primates are being used to develop a cortical visual prosthesis for the blind. We use the properties of electrical microstimulation of striate cortex (area V1) of macaque monkeys to make inferences about phosphene induction. Our analysis is based on well-established properties of V1: retino-cortical magnification factor, receptive-field size, and the characteristics of hypercolumns. We argue that phosphene size is dependent on the amount of current delivered to V1 and on the retino-cortical magnification factor. We suggest that to improve the correspondence between the site of stimulation within V1 and the visual field location of an elicited phosphene both eyes must be put under experimental control given that phosphene location is retinocentric and given that the vergence angle between the eyes might affect the position of a phosphene in depth. Knowing how electrical microstimulation interacts with cortical tissue to evoke percepts in behaving macaque monkeys is fundamental to the establishment of an effective cortical visual prosthesis for the blind.
Figures
References
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J. Neurophysiol. 1982;48:217–237. - PubMed
-
- Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, Schmidt EM. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Med. Biol. Eng. Comput. 1990;28:257–259. - PubMed
-
- Bartlett JR, DeYoe EA, Doty RW, Lee BB, Lewine JD, Negrão N, Overman WH., Jr. Psychophysics of electrical stimulation of striate cortex in macaques. J. Neurophysiol. 2005;94:3430–3442. - PubMed
-
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J. Neurophysiol. 2002;87:478–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

