Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17
- PMID: 17174564
- PMCID: PMC2692377
- DOI: 10.1016/j.mcn.2006.11.001
Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17
Abstract
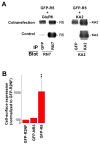
Correct glutamate receptor localization in neurons is crucial for neurotransmission in the brain. Here we investigated the mechanisms underlying localization of kainate GluR5 receptors to dendrites in cultured hippocampal neurons. We find that the GluR5 distribution depends on association with GluR6 and KA2 subunits. The GluR5 subunit was expressed in distal dendrites only when GluR6 and KA2 subunits were present, whereas it was restricted to proximal dendrites in the absence of these subunits. The overlap between GluR5 distribution and the organization of microtubules in dendrites led us to examine whether KIF17, a microtubule motor protein expressed in distal dendrites, is involved in GluR5 localization to distal dendrites. We show here, for the first time that the microtubule motor protein KIF17 interacts with GluR6 and KA2 subunits and is required for GluR5 localization to distal dendrites, defining a novel mechanism that controls receptor localization in neurons.
Figures




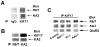

References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Bah J, Quach H, Ebstein RP, Segman RH, Melke J, Jamain S, Rietschel M, Modai I, Kanas K, Karni O, Lerer B, Gourion D, Krebs MO, Etain B, Schurhoff F, Szoke A, Leboyer M, Bourgeron T. Maternal transmission disequilibrium of the glutamate receptor GRIK2 in schizophrenia. Neuroreport. 2004;15:1987–1991. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

