Targeted inactivation of synaptic HRG4 (UNC119) causes dysfunction in the distal photoreceptor and slow retinal degeneration, revealing a new function
- PMID: 17174953
- PMCID: PMC1820979
- DOI: 10.1016/j.exer.2006.10.016
Targeted inactivation of synaptic HRG4 (UNC119) causes dysfunction in the distal photoreceptor and slow retinal degeneration, revealing a new function
Abstract
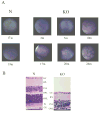
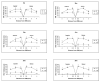
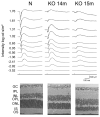
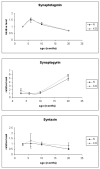
HRG4 (UNC119) is a photoreceptor protein predominantly localized to the photoreceptor synapses and to the inner segments to a lesser degree. A heterozygous truncation mutation in HRG4 was found in a patient with late onset cone-rod dystrophy, and a transgenic (TG) mouse expressing the identical mutant protein developed late onset retinal degeneration, confirming the pathogenic potential of HRG4. Recently, the dominant negative pathogenic mechanism in the TG model was shown to involve increased affinity of the truncated mutant HRG4 for its target, ARL2, which leads to a delayed decrease in its downstream target, mitochondrial ANT1, mitochondrial stress, synaptic degeneration, trans-synaptic degeneration, and whole photoreceptor degeneration by apoptosis. In this study, the mouse HRG4 (MRG4) gene was cloned and targeted to construct a knock-out (KO) mouse model of HRG4 in order to study the effects of completely inactivating this protein. The KO model was examined by genomic Southern blotting, Western blotting, immunofluorescence, funduscopy, LM and EM histopathology, ERG, and TUNEL analyses. The KO model developed a slowly progressive retinal degeneration, characterized by mottling in the fundus, mild thinning of the photoreceptor layer, and increase in apoptosis as early as 6 months, dramatic acceleration at approximately 17 months, and virtual obliteration of the photoreceptors by 20 months. When compared to retinal degeneration in the TG model, significant differences existed in the KO consisting of more severe and early photoreceptor death without evidence of early synaptic and trans-synaptic degeneration as seen in the TG, confirmed by LM and EM histopathology, ERG, and Western blotting of synaptic proteins. The results indicated a dysfunction in the KO outside the synapses in the distal end of photoreceptors where MRG4 is also localized. Differences in the phenotypes of retinal degeneration in the KO and TG models reflect a dysfunction in the two opposite ends of photoreceptors, i.e., the distal inner/outer segments and proximal synapses, respectively, indicating a second function of MRG4 in the distal photoreceptor and dual functionality of MRG4. Thus, inactivation of MRG4 by gene targeting resulted in a retinal degeneration phenotype quite different from that previously seen in the TG, attesting to the multiplicity of MRG4 function, in addition to the importance of this protein for normal retinal function. These models will be useful in elucidating the functions of HRG4/MRG4 and the mechanism of slow retinal degeneration.
Figures




Similar articles
-
Truncation mutation in HRG4 (UNC119) leads to mitochondrial ANT-1-mediated photoreceptor synaptic and retinal degeneration by apoptosis.Invest Ophthalmol Vis Sci. 2006 Apr;47(4):1281-92. doi: 10.1167/iovs.05-0493. Invest Ophthalmol Vis Sci. 2006. PMID: 16565359
-
Changes in retinal synaptic proteins in the transgenic model expressing a mutant HRG4 (UNC119).Invest Ophthalmol Vis Sci. 2002 Feb;43(2):308-13. Invest Ophthalmol Vis Sci. 2002. PMID: 11818371
-
HRG4 (UNC119) mutation found in cone-rod dystrophy causes retinal degeneration in a transgenic model.Invest Ophthalmol Vis Sci. 2000 Oct;41(11):3268-77. Invest Ophthalmol Vis Sci. 2000. PMID: 11006213
-
Retinal degeneration mutants in the mouse.Vision Res. 2002 Feb;42(4):517-25. doi: 10.1016/s0042-6989(01)00146-8. Vision Res. 2002. PMID: 11853768 Review.
-
Functional changes in inner retinal neurons in animal models of photoreceptor degeneration.Adv Exp Med Biol. 2010;664:525-32. doi: 10.1007/978-1-4419-1399-9_60. Adv Exp Med Biol. 2010. PMID: 20238055 Review.
Cited by
-
Unc119 gene deletion partially rescues the GRK1 transport defect of Pde6d (- /-) cones.Adv Exp Med Biol. 2014;801:487-93. doi: 10.1007/978-1-4614-3209-8_62. Adv Exp Med Biol. 2014. PMID: 24664735 Free PMC article.
-
ARF family GTPases with links to cilia.Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C404-C418. doi: 10.1152/ajpcell.00188.2020. Epub 2020 Jun 10. Am J Physiol Cell Physiol. 2020. PMID: 32520609 Free PMC article. Review.
-
Transducin Partners Outside the Phototransduction Pathway.Front Cell Neurosci. 2020 Oct 14;14:589494. doi: 10.3389/fncel.2020.589494. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33173469 Free PMC article.
-
Expression and subcellular distribution of UNC119a, a protein partner of transducin α subunit in rod photoreceptors.Cell Signal. 2013 Jan;25(1):341-8. doi: 10.1016/j.cellsig.2012.10.005. Epub 2012 Oct 13. Cell Signal. 2013. PMID: 23072788 Free PMC article.
-
Arf-like Protein 3 (ARL3) Regulates Protein Trafficking and Ciliogenesis in Mouse Photoreceptors.J Biol Chem. 2016 Mar 25;291(13):7142-55. doi: 10.1074/jbc.M115.710954. Epub 2016 Jan 25. J Biol Chem. 2016. PMID: 26814127 Free PMC article.
References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
-
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. - PubMed
-
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN, Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci U S A. 2000;97:13913–13918. - PMC - PubMed
-
- Calvert PD, Govardovskii VI, Krasnoperova N, Anderson RE, Lem J, Makino CL. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

