FT-IR imaging of native and tissue-engineered bone and cartilage
- PMID: 17175021
- PMCID: PMC1892909
- DOI: 10.1016/j.biomaterials.2006.11.043
FT-IR imaging of native and tissue-engineered bone and cartilage
Abstract
Fourier transform infrared (FT-IR) imaging and microspectroscopy have been extensively applied to the analyses of tissues in health and disease. Spatially resolved mid-IR data has provided insights into molecular changes that occur in diseases of connective or collagen-based tissues, including, osteoporosis, osteogenesis imperfecta, osteopetrosis and pathologic calcifications. These techniques have also been used to probe chemical changes associated with load, disuse, and micro-damage in bone, and with degradation and repair in cartilage. This review summarizes the applications of FT-IR microscopy and imaging for analyses of bone and cartilage in healthy and diseased tissues, and illustrates the application of these techniques for the characterization of tissue-engineered bone and cartilage.
Figures

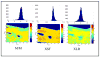
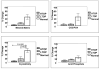
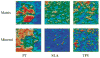
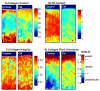
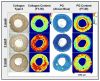

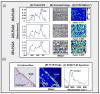
References
-
- Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt. 2005;10:031102–031106. - PubMed
-
- Carden A, Morris MD. Application of vibrational spectroscopy to the study of mineralized tissues. J Biomed Opt. 2000;5:259–268. - PubMed
-
- Miller LM, Dumas P. Chemical imaging of biological tissue with synchrotron infrared light. Biochim Biophys Acta. 2006;1758:846–857. - PubMed
-
- West PA, Bostrom MP, Torzilli PA, Camacho NP. Fourier transform infrared spectral analysis of degenerative cartilage: an infrared fiber optic probe and imaging study. Appl Spectrosc. 2004;58:376–81. - PubMed
-
- Barer R, Cole ARH, Thompson HW. Infrared spectroscopy with the reflecting microscope in physics, chemistry and biology. Nature. 1949;163:198–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EB00744/EB/NIBIB NIH HHS/United States
- AR043125/AR/NIAMS NIH HHS/United States
- R01 AR056145/AR/NIAMS NIH HHS/United States
- AR48337/AR/NIAMS NIH HHS/United States
- R01 AR041325/AR/NIAMS NIH HHS/United States
- DE04141/DE/NIDCR NIH HHS/United States
- R01 AR048337/AR/NIAMS NIH HHS/United States
- P30 AR046121/AR/NIAMS NIH HHS/United States
- R37 DE004141/DE/NIDCR NIH HHS/United States
- C06 RR012538/RR/NCRR NIH HHS/United States
- AR037661/AR/NIAMS NIH HHS/United States
- R01 EB000744/EB/NIBIB NIH HHS/United States
- R01 DE004141/DE/NIDCR NIH HHS/United States
- R01 AR037661/AR/NIAMS NIH HHS/United States
- C06-RR12538-01/RR/NCRR NIH HHS/United States
- AR046121/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

