Genetic dependence of cochlear cells and structures injured by noise
- PMID: 17175124
- PMCID: PMC1809471
- DOI: 10.1016/j.heares.2006.11.005
Genetic dependence of cochlear cells and structures injured by noise
Abstract
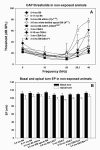
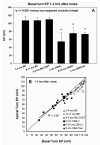
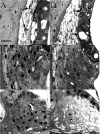
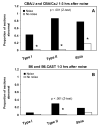
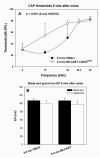
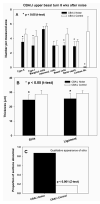
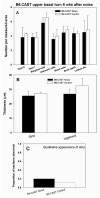
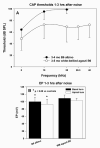
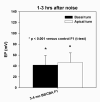
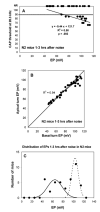
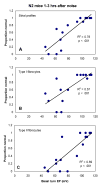
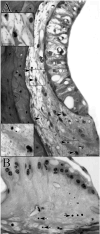
The acute and permanent effects of a single damaging noise exposure were compared in CBA/J, C57BL/6 (B6), and closely related strains of mice. Two hours of broadband noise (4-45 kHz) at 110 dB SPL led to temporary reduction in the endocochlear potential (EP) of CBA/J and CBA/CaJ (CBA) mice and acute cellular changes in cochlear stria vascularis and spiral ligament. For the same exposure, B6 mice showed no EP reduction and little of the pathology seen in CBA. Eight weeks after exposure, all mice showed a normal EP, but only CBA mice showed injury and cell loss in cochlear lateral wall, despite the fact that B6 sustained larger permanent threshold shifts. Examination of noise injury in B6 congenics carrying alternate alleles of genes encoding otocadherin (Cdh23), agouti protein, and tyrosinase (albinism) indicated that none of these loci can account for the strain differences observed. Examination of CBA x B6 F1 mice and N2 backcross mice to B6 further indicated that susceptibility to noise-related EP reduction and associated cell pathology are inherited in an autosomal dominant manner, and are established by one or a few large effect quantitative trait loci. Findings support a common genetic basis for an entire constellation of noise-related cochlear pathologies in cochlear lateral wall and spiral limbus. Even within species, cellular targets of acute and permanent cochlear noise injury may vary with genetic makeup.
Figures


Similar articles
-
Mechanisms and genes in human strial presbycusis from animal models.Brain Res. 2009 Jun 24;1277:70-83. doi: 10.1016/j.brainres.2009.02.079. Epub 2009 Mar 12. Brain Res. 2009. PMID: 19285967 Free PMC article. Review.
-
Different cellular and genetic basis of noise-related endocochlear potential reduction in CBA/J and BALB/cJ mice.J Assoc Res Otolaryngol. 2011 Feb;12(1):45-58. doi: 10.1007/s10162-010-0238-z. Epub 2010 Oct 5. J Assoc Res Otolaryngol. 2011. PMID: 20922451 Free PMC article.
-
A major effect QTL on chromosome 18 for noise injury to the mouse cochlear lateral wall.Hear Res. 2010 Feb;260(1-2):47-53. doi: 10.1016/j.heares.2009.11.006. Epub 2009 Nov 12. Hear Res. 2010. PMID: 19913606 Free PMC article.
-
The endocochlear potential as an indicator of reticular lamina integrity after noise exposure in mice.Hear Res. 2018 Apr;361:138-151. doi: 10.1016/j.heares.2018.01.015. Epub 2018 Feb 1. Hear Res. 2018. PMID: 29426600 Free PMC article.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
Cited by
-
Mechanisms and genes in human strial presbycusis from animal models.Brain Res. 2009 Jun 24;1277:70-83. doi: 10.1016/j.brainres.2009.02.079. Epub 2009 Mar 12. Brain Res. 2009. PMID: 19285967 Free PMC article. Review.
-
A critical evaluation of "leakage" at the cochlear blood-stria-barrier and its functional significance.Front Mol Neurosci. 2024 Feb 29;17:1368058. doi: 10.3389/fnmol.2024.1368058. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38486963 Free PMC article. Review.
-
Age-related auditory pathology in the CBA/J mouse.Hear Res. 2008 Sep;243(1-2):87-94. doi: 10.1016/j.heares.2008.06.001. Epub 2008 Jun 7. Hear Res. 2008. PMID: 18573325 Free PMC article.
-
Protection by low-dose kanamycin against noise-induced hearing loss in mice: dependence on dosing regimen and genetic background.Hear Res. 2011 Oct;280(1-2):141-7. doi: 10.1016/j.heares.2011.05.007. Epub 2011 May 27. Hear Res. 2011. PMID: 21645602 Free PMC article.
-
Down-regulation of AMPK signaling pathway rescues hearing loss in TFB1 transgenic mice and delays age-related hearing loss.Aging (Albany NY). 2020 Apr 2;12(7):5590-5611. doi: 10.18632/aging.102977. Epub 2020 Apr 2. Aging (Albany NY). 2020. PMID: 32240104 Free PMC article.
References
-
- Axelsson A, Borg E, Hornstrand C. Noise effects on the cochlear vasculature in normotensive and spontaneously hypertensive rats. Acta Otolaryngol. 1983;96:215–225. - PubMed
-
- Barrenas ML, Holgers KM. Ototoxic interaction between noise and pheomelanin: Distortion product otoacoustic emissions after acoustical trauma in chloroquine-treated red, black, and albino guinea pigs. Audiology. 2000;39:238–246. - PubMed
-
- Barrenas ML, Lindgren F. The influence of eye color on susceptibility to TTS in humans. Br J Audiol. 1991;25:303–307. - PubMed
-
- Barrenas M. Hair cell loss from acoustic trauma in chloroquine-treated red, black and albino guinea pigs. Audiology. 1997;36:187–201. - PubMed
-
- Beagley HA. Acoustic trauma in the guinea pig. I Electrophysiology and Histology. Acta Otolaryngol. 1965;60:437–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

