Comparison of treatment effects between animal experiments and clinical trials: systematic review
- PMID: 17175568
- PMCID: PMC1781970
- DOI: 10.1136/bmj.39048.407928.BE
Comparison of treatment effects between animal experiments and clinical trials: systematic review
Abstract
Objective: To examine concordance between treatment effects in animal experiments and clinical trials. Study design Systematic review.
Data sources: Medline, Embase, SIGLE, NTIS, Science Citation Index, CAB, BIOSIS.
Study selection: Animal studies for interventions with unambiguous evidence of a treatment effect (benefit or harm) in clinical trials: head injury, antifibrinolytics in haemorrhage, thrombolysis in acute ischaemic stroke, tirilazad in acute ischaemic stroke, antenatal corticosteroids to prevent neonatal respiratory distress syndrome, and bisphosphonates to treat osteoporosis. Review methods Data were extracted on study design, allocation concealment, number of randomised animals, type of model, intervention, and outcome.
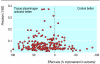
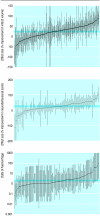
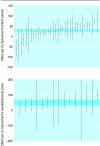
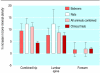
Results: Corticosteroids did not show any benefit in clinical trials of treatment for head injury but did show a benefit in animal models (pooled odds ratio for adverse functional outcome 0.58, 95% confidence interval 0.41 to 0.83). Antifibrinolytics reduced bleeding in clinical trials but the data were inconclusive in animal models. Thrombolysis improved outcome in patients with ischaemic stroke. In animal models, tissue plasminogen activator reduced infarct volume by 24% (95% confidence interval 20% to 28%) and improved neurobehavioural scores by 23% (17% to 29%). Tirilazad was associated with a worse outcome in patients with ischaemic stroke. In animal models, tirilazad reduced infarct volume by 29% (21% to 37%) and improved neurobehavioural scores by 48% (29% to 67%). Antenatal corticosteroids reduced respiratory distress and mortality in neonates whereas in animal models respiratory distress was reduced but the effect on mortality was inconclusive (odds ratio 4.2, 95% confidence interval 0.85 to 20.9). Bisphosphonates increased bone mineral density in patients with osteoporosis. In animal models the bisphosphonate alendronate increased bone mineral density compared with placebo by 11.0% (95% confidence interval 9.2% to 12.9%) in the combined results for the hip region. The corresponding treatment effect in the lumbar spine was 8.5% (5.8% to 11.2%) and in the combined results for the forearms (baboons only) was 1.7% (-1.4% to 4.7%).
Conclusions: Discordance between animal and human studies may be due to bias or to the failure of animal models to mimic clinical disease adequately.
Conflict of interest statement
Competing interests: IR was an investigator in the corticosteroid randomisation after significant head injury trial. The trial was funded by the UK Medical Research Council. Pharmacia and Upjohn (Pfizer from 2003) provided the Medical Research Council with the methylprednisolone (free of charge) needed for the trial, a grant in aid for preparation of the placebo, and support for collaborators' meetings. PS is co-chief investigator of the third international stroke trial, testing intravenous recombinant tissue plasminogen activator in acute ischaemic stroke; the start-up phase (completed in 2005) of this trial was supported by Boehringer Ingelheim, the manufacturers of tissue plasminogen activator, a donation of drug and placebo for the first 300 patients. The current phase of the trial is supported by the Medical Research Council and the Health Foundation. None of the authors have any relevant competing financial interests.
Figures

Comment in
-
Translating animal research into clinical benefit.BMJ. 2007 Jan 27;334(7586):163-4. doi: 10.1136/bmj.39104.362951.80. BMJ. 2007. PMID: 17255568 Free PMC article.
-
A broader view of animal research.BMJ. 2007 Feb 10;334(7588):274. doi: 10.1136/bmj.39115.390984.1F. BMJ. 2007. PMID: 17289697 Free PMC article. No abstract available.
References
-
- MORI. Research study conducted for the Coalition for Medical Progress. The use of animals in medical research. Mar-May 2002. www.mori.com/polls/2002/pdf/cmp.pdf
-
- Croce P. Vivisection or science? An investigation into testing drugs and safeguarding health London: Zed Books, 1999
-
- Sandercock P, Roberts I. Systematic reviews of animal experiments. Lancet 2002;360:586. - PubMed
-
- Macleod M, Sandercock P. Can systematic reviews help animal experimental work? RDS News winter 2005
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
