Cytoskeletal tension regulates both expression and degradation of h2-calponin in lung alveolar cells
- PMID: 17176089
- PMCID: PMC1764619
- DOI: 10.1021/bi061718f
Cytoskeletal tension regulates both expression and degradation of h2-calponin in lung alveolar cells
Abstract
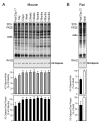
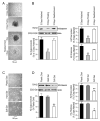
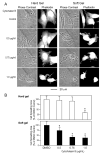
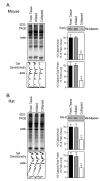
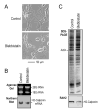
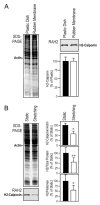
Calponin is an actin filament-associated regulatory protein, and its h2 isoform is expressed in lung alveolar epithelial cells under postnatal upregulation during lung development corresponding to the commencement of respiratory expansion. Consistent with this correlation to mechanical tension, the expression of h2-calponin in alveolar cells is dependent on substrate stiffness and cytoskeleton tension. The function of h2-calponin in the stability of actin cytoskeleton implicates a role in balancing the strength and compliance of alveoli. An interesting finding is a rapid degradation of h2-calponin in lung after prolonged deflation, which is prevented by inflation of the lung to the in situ expanded volume. Decreasing mechanical tension in cultured alveolar cells by reducing the dimension of culture matrix reproduced the degradation of h2-calponin. Inhibition of myosin II ATPase also resulted in the degradation of h2-calponin in alveolar cells, showing a determining role of the tension in the actin cytoskeleton. Alveolar cells statically cultured on silicon rubber membrane build high tension in the cytoskeleton corresponding to a high expression of h2-calponin. Chronic cyclic stretching of cells on the membrane did not increase but decreased the expression of h2-calponin. This finding suggests that when cellular structure adapts to the stretched dimension, cyclic relaxations periodically release cytoskeleton tension and lower the total amount of tension that the cell senses over time. Therefore, the isometric tension, other than tension dynamics, determines the expression of h2-calponin. The tension regulation of h2-calponin synthesis and degradation demonstrates a novel mechanical regulation of cellular biochemistry.
Figures






References
-
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. - PubMed
-
- Eckes B, Krieg T. Regulation of connective tissue homeostasis in the skin by mechanical forces. Clin Exp Rheumatol. 2004;22:S73–S76. - PubMed
-
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. - PubMed
-
- Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36:631–643. - PubMed
-
- Walker JL, Fournier AK, Assoian RK. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine Growth Factor Rev. 2005;16:395–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

