Interaction of heparin with two synthetic peptides that neutralize the anticoagulant activity of heparin
- PMID: 17176096
- PMCID: PMC2527756
- DOI: 10.1021/bi061346a
Interaction of heparin with two synthetic peptides that neutralize the anticoagulant activity of heparin
Abstract
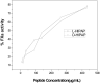
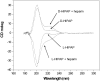
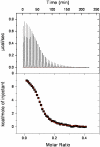
Two synthetic analogues of the heparin-binding domain of heparin/heparan sulfate-interacting protein (Ac-SRGKAKVKAKVKDQTK-NH2) and the all-d-amino acid version of the same peptide (l-HIPAP and d-HIPAP, respectively) were synthesized, and their efficacy as agents for neutralization of the anticoagulant activity of heparin was assayed. The two analogue peptides were found to be equally effective for neutralization of the anticoagulant activity of heparin, as measured by restoration of the activity of serine protease factor Xa by the Coatest heparin method. The finding that l-HIPAP and d-HIPAP are equally effective suggests that d-amino acid peptides show promise as proteolytically stable therapeutic agents for neutralization of the anticoagulant activity of heparin. The interaction of l-HIPAP and d-HIPAP with heparin was characterized by 1H NMR, isothermal titration calorimetry (ITC), and heparin affinity chromatography. The two peptides were found to interact identically with heparin. Analysis of the dependence of heparin-peptide binding constants on Na+ concentration by counterion condensation theory indicates that, on average, 2.35 Na+ ions are displaced from heparin per peptide molecule bound and one peptide molecule binds per hexasaccharide segment of heparin. The analysis also indicates that both ionic and nonionic interactions contribute to the binding constant, with the ionic contribution decreasing as the Na+ concentration increases.
Figures


References
-
- Rodén L. Highlights in the History of Heparin. In: Lane DA, Lindahl U, editors. Heparin: Chemical and Biological Properties, Clinical Applications. CRC Press, Inc.; Boca Raton, FL: 1989. pp. 1–23.
-
- Rabenstein DL. Heparin and heparin sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. - PubMed
-
- Olivercrona T, Bengtsson-Olivercrona G. Heparin and Lipases. In: Lane DA, Lindahl U, editors. Heparin: Chemical and Biological Properties, Clinical Applications. CRC Press, Inc.; Boca Raton, FL: 1989. pp. 335–361.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

