Secretion and properties of a hybrid Kluyveromyces lactis-Aspergillus niger beta-galactosidase
- PMID: 17176477
- PMCID: PMC1764428
- DOI: 10.1186/1475-2859-5-41
Secretion and properties of a hybrid Kluyveromyces lactis-Aspergillus niger beta-galactosidase
Abstract
Background: The beta-galactosidase from Kluyveromyces lactis is a protein of outstanding biotechnological interest in the food industry and milk whey reutilization. However, due to its intracellular nature, its industrial production is limited by the high cost associated to extraction and downstream processing. The yeast-system is an attractive method for producing many heterologous proteins. The addition of a secretory signal in the recombinant protein is the method of choice to sort it out of the cell, although biotechnological success is not guaranteed. The cell wall acting as a molecular sieve to large molecules, culture conditions and structural determinants present in the protein, all have a decisive role in the overall process. Protein engineering, combining domains of related proteins, is an alternative to take into account when the task is difficult. In this work, we have constructed and analyzed two hybrid proteins from the beta-galactosidase of K. lactis, intracellular, and its Aspergillus niger homologue that is extracellular. In both, a heterologous signal peptide for secretion was also included at the N-terminus of the recombinant proteins. One of the hybrid proteins obtained has interesting properties for its biotechnological utilization.
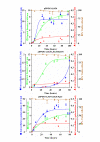
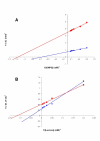
Results: The highest levels of intracellular and extracellular beta-galactosidase were obtained when the segment corresponding to the five domain of K. lactis beta-galactosidase was replaced by the corresponding five domain of the A. niger beta-galactosidase. Taking into account that this replacement may affect other parameters related to the activity or the stability of the hybrid protein, a thoroughly study was performed. Both pH (6.5) and temperature (40 degrees C) for optimum activity differ from values obtained with the native proteins. The stability was higher than the corresponding to the beta-galactosidase of K. lactis and, unlike this, the activity of the hybrid protein was increased by the presence of Ni2+. The affinity for synthetic (ONPG) or natural (lactose) substrates was higher in the hybrid than in the native K. lactis beta-galactosidase. Finally, a structural-model of the hybrid protein was obtained by homology modelling and the experimentally determined properties of the protein were discussed in relation to it.
Conclusion: A hybrid protein between K. lactis and A. niger beta-galactosidases was constructed that increases the yield of the protein released to the growth medium. Modifications introduced in the construction, besides to improve secretion, conferred to the protein biochemical characteristics of biotechnological interest.
Figures





References
-
- González Siso MI. The biotechnological utilization of cheese whey: a review. Biores Technol. 1996;57:1–11. doi: 10.1016/0960-8524(96)00036-3. - DOI
-
- Becerra M, Cerdán ME, González Siso MI. Microescale purification of the enzyme β-galactosidase from Kluyveromyces lactis reveals that dimeric and tetrameric forms are active. Biotechnol Techniques. 1998;12:253–256. doi: 10.1023/A:1008885827560. - DOI
LinkOut - more resources
Full Text Sources

