Diindolylquinoxalines: effective indole-based receptors for phosphate anion
- PMID: 17177398
- PMCID: PMC2631397
- DOI: 10.1021/ja067720b
Diindolylquinoxalines: effective indole-based receptors for phosphate anion
Abstract
The synthesis of a new series of 2,3-diindol-3'yl quinoxalines (DIQ), as well as a comparison of their anion recognition properties to those of our previously reported pyrrole based sensors, 2,3-dipyrrol-2'yl quinoxalines, is reported. To the best of our knowledge, this new DIQ system represents the first example of a free-standing indole-based small molecule receptor for which evidence of anion binding is available both in solution and in the solid-state. It also provides one of the few structurally characterized neutral receptor-dihydrogen phosphate complexes. This work thus serves to demonstrate the utility of indoles as an anion recognition motif.
Figures


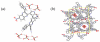
Similar articles
-
Tetrathiafulvalene diindolylquinoxaline: a dual signaling anion receptor with phosphate selectivity.Chem Commun (Camb). 2010 Nov 7;46(41):7745-7. doi: 10.1039/c0cc02934c. Epub 2010 Sep 20. Chem Commun (Camb). 2010. PMID: 20856940 Free PMC article.
-
Synthesis and characterization of indolocarbazole-quinoxalines with flat rigid structure for sensing fluoride and acetate anions.Org Biomol Chem. 2008 May 21;6(10):1751-5. doi: 10.1039/b801447g. Epub 2008 Mar 28. Org Biomol Chem. 2008. PMID: 18452009
-
Metal-induced pre-organisation for anion recognition in a neutral platinum-containing receptor.Chem Commun (Camb). 2009 Nov 7;(41):6279-81. doi: 10.1039/b912942a. Epub 2009 Sep 4. Chem Commun (Camb). 2009. PMID: 19826694
-
Tricyclic quinoxalinediones, aza-kynurenic acids, and indole-2-carboxylic acids as in vivo active NMDA-glycine antagonists.Curr Top Med Chem. 2006;6(7):733-45. doi: 10.2174/156802606776894500. Curr Top Med Chem. 2006. PMID: 16719813 Review.
-
Structure, function and interfacial allosterism in phospholipase A2: insight from the anion-assisted dimer.Arch Biochem Biophys. 2005 Jan 1;433(1):96-106. doi: 10.1016/j.abb.2004.08.013. Arch Biochem Biophys. 2005. PMID: 15581569 Review.
Cited by
-
A ferrocene-quinoxaline derivative as a highly selective probe for colorimetric and redox sensing of toxic mercury(II) cations.Sensors (Basel). 2010;10(12):11311-21. doi: 10.3390/s101211311. Epub 2010 Dec 10. Sensors (Basel). 2010. PMID: 22163528 Free PMC article.
-
Exploring nonlinear optical properties in a hybrid dihydrogen phosphate system: an experimental and theoretical approach.J Mol Model. 2024 Apr 26;30(5):151. doi: 10.1007/s00894-024-05936-x. J Mol Model. 2024. PMID: 38668860
-
Anion Cluster: Assembly of Dihydrogen Phosphates for the Formation of a Cyclic Anion Octamer.Cryst Growth Des. 2012 Feb 1;12(2):567-571. doi: 10.1021/cg201464k. Cryst Growth Des. 2012. PMID: 22435043 Free PMC article.
-
Tetrathiafulvalene diindolylquinoxaline: a dual signaling anion receptor with phosphate selectivity.Chem Commun (Camb). 2010 Nov 7;46(41):7745-7. doi: 10.1039/c0cc02934c. Epub 2010 Sep 20. Chem Commun (Camb). 2010. PMID: 20856940 Free PMC article.
-
Crystal structure of a host-guest complex of the tris-urea receptor, 3-(4-nitro-phen-yl)-1,1-bis-{2-[3-(4-nitro-phen-yl)ureido]eth-yl}urea, that encapsulates hydrogen-bonded chains of di-hydrogen phosphate anions with separate tetra-n-butyl-ammonium counter-ions.Acta Crystallogr E Crystallogr Commun. 2019 Feb 5;75(Pt 3):319-323. doi: 10.1107/S2056989019001336. eCollection 2019 Mar 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 30867940 Free PMC article.
References
-
- Saenger W. Principles of Nucleic Acid Structure. New York; Springer Verlag: 1988.
-
- Hruska K, Teitlebaum S. New Engl. J. Med. 1995;333:166–175. - PubMed
- Robinson C. Dental Digest. 2000:1–3.
-
- Sessler J, Gale PA, Cho W-S. Anion Receptor Chemistry. Cambridge, UK: 2006. The Royal Society of Chemistry.
- Beer PD, Gale PA. Angew. Chem., Int. Ed. 2001;41:486–516. - PubMed
-
- Kendo S-I, Hiraoka Y, Kurumatani N, Yano Y. Chem.Comm. 2005:1720–1722. - PubMed
- Seong HR, Kim D-S, Kim S-G, Choi H-J, Ahn KH. Tetrahedron Lett. 2004;45:723–727.
- Chmielewski MJ, Charon M, Jurczak J. Org. Lett. 2004;6:3501–3504. - PubMed
- Han MS, Kim DH. Angew. Chem., Int. Ed. 2002;41:3809–3811. - PubMed
- Kuo L-J, Liao J-H, Chen C-T, Huang C-H, Chen C-S, Fang J-M. Org. Lett. 2003;5:1821–1824. - PubMed
- Tobey SL, Anslyn EV. Org. Lett. 2003;5:2029–2031. - PubMed
- Anzenbacher P, Jursikova K, Sessler JL. J. Am. Chem. Soc. 2000;122:9350–9351.
- Beer PD, Dickson CAP, Fletcher N, Goulden AJ, Grieve A, Hodacova J, Wear T. J. Chem. Soc., Chem. Commun. 1993:828–830.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

