A central source of movement variability
- PMID: 17178410
- PMCID: PMC1941679
- DOI: 10.1016/j.neuron.2006.10.034
A central source of movement variability
Abstract
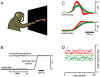
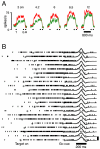
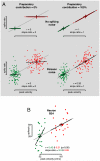
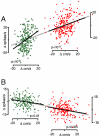
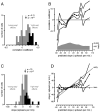
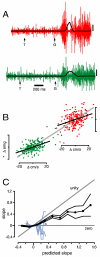
Movements are universally, sometimes frustratingly, variable. When such variability causes error, we typically assume that something went wrong during the movement. The same assumption is made by recent and influential models of motor control. These posit that the principal limit on repeatable performance is neuromuscular noise that corrupts movement as it occurs. An alternative hypothesis is that movement variability arises before movements begin, during motor preparation. We examined this possibility directly by recording the preparatory activity of single cortical neurons during a highly practiced reach task. Small variations in preparatory neural activity were predictive of small variations in the upcoming reach. Effect magnitudes were such that at least half of the observed movement variability likely had its source during motor preparation. Thus, even for a highly practiced task, the ability to repeatedly plan the same movement limits our ability to repeatedly execute the same movement.
Figures


References
-
- Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol. 2006a - PubMed
-
- Cisek P. Preparing for speed. Focus on: ‘Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach’. J Neurophysiol. 2006 - PubMed
-
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

