Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation
- PMID: 17178784
- PMCID: PMC1828547
- DOI: 10.1128/IAI.01315-06
Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation
Abstract
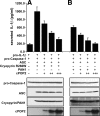
Pyrin domain (PYD) proteins have recently emerged as important signaling molecules involved in the development of innate immunity against intracellular pathogens through activation of inflammatory mediator pathways. ASC is the central adaptor protein, which links pathogen recognition by PYD-containing pathogen recognition receptors, known as PYD-Nod-like receptors (NLR), PAN, PYPAF, NALP, Nod, and Caterpiller proteins, to the activation of downstream effectors, including activation of caspase-1 and NF-kappaB. Activation of these effectors occurs when specific protein complexes, known as inflammasomes, are formed. PYD signal transduction leads to inflammasome assembly and activation of specific effector proteins. It is modulated by a cellular PYD-only protein (cPOP1), which binds to ASC and interferes with the recruitment of ASC to activated PYD-NLRs. Here we describe the identification and characterization of a second cellular POP (cPOP2), which shows highest homology to the PYD of PAN1. cPOP2 binds to ASC and PAN1, thereby blocking formation of cryopyrin and PAN1-containing inflammasomes, activation of caspase-1, and subsequent processing and secretion of bioactive interleukin-1beta. Existence of a second cPOP provides additional insights into inflammasome formation and suggests that POPs might be a common regulatory mechanism to "fine-tune" the activity of specific PYD-NLR family protein-containing inflammasomes.
Figures




References
-
- Agostini, L., F. Martinon, K. Burns, M. F. McDermott, P. N. Hawkins, and J. Tschopp. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20:319-325. - PubMed
-
- Bruey, J. M., N. Bruey-Sedano, R. Newman, S. Chandler, C. Stehlik, and J. C. Reed. 2004. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J. Biol. Chem. 279:51897-51907. - PubMed
-
- Brydges, S., and D. L. Kastner. 2006. The systemic autoinflammatory diseases: inborn errors of the innate immune system. Curr. Top. Microbiol. Immunol. 305:127-160. - PubMed
-
- Condorelli, G., G. Vigliotta, A. Cafieri, A. Trencia, P. Andalo, F. Oriente, C. Miele, M. Caruso, P. Formisano, and F. Beguinot. 1999. PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene 18:4409-4415. - PubMed
-
- Conway, K. E., B. B. McConnell, C. E. Bowring, C. D. Donald, S. T. Warren, and P. M. Vertino. 2000. TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res. 60:6236-6242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

