Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels
- PMID: 17178908
- PMCID: PMC2064700
- DOI: 10.1083/jcb.200608073
Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels
Abstract
The voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane mediates metabolic flow, Ca(2+), and cell death signaling between the endoplasmic reticulum (ER) and mitochondrial networks. We demonstrate that VDAC1 is physically linked to the endoplasmic reticulum Ca(2+)-release channel inositol 1,4,5-trisphosphate receptor (IP(3)R) through the molecular chaperone glucose-regulated protein 75 (grp75). Functional interaction between the channels was shown by the recombinant expression of the ligand-binding domain of the IP(3)R on the ER or mitochondrial surface, which directly enhanced Ca(2+) accumulation in mitochondria. Knockdown of grp75 abolished the stimulatory effect, highlighting chaperone-mediated conformational coupling between the IP(3)R and the mitochondrial Ca(2+) uptake machinery. Because organelle Ca(2+) homeostasis influences fundamentally cellular functions and death signaling, the central location of grp75 may represent an important control point of cell fate and pathogenesis.
Figures
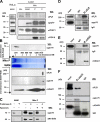
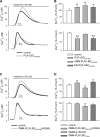


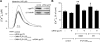
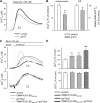
References
-
- Berridge, M.J., M.D. Bootman, and H.L. Roderick. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529. - PubMed
-
- Bosanac, I., T. Michikawa, and K. Mikoshiba. 2004. Structural insights into the regulatory mechanism of IP3 receptor. Biochim. Biophys. Acta. 1742:89–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

