Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors
- PMID: 17178910
- PMCID: PMC2064703
- DOI: 10.1083/jcb.200604073
Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors
Abstract
STAT transcription factors are tyrosine phosphorylated upon cytokine stimulation and enter the nucleus to activate target genes. We show that Rac1 and a GTPase-activating protein, MgcRacGAP, bind directly to p-STAT5A and are required to promote its nuclear translocation. Using permeabilized cells, we find that nuclear translocation of purified p-STAT5A is dependent on the addition of GTP-bound Rac1, MgcRacGAP, importin alpha, and importin beta. p-STAT3 also enters the nucleus via this transport machinery, and mutant STATs lacking the MgcRacGAP binding site do not enter the nucleus even after phosphorylation. We conclude that GTP-bound Rac1 and MgcRacGAP function as a nuclear transport chaperone for activated STATs.
Figures


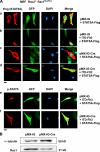



References
-
- Blagoev, B., I. Kratchmarova, S.E. Ong, M. Nielsen, L.J. Foster, and M. Mann. 2003. A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat. Biotechnol. 21:315–318. - PubMed
-
- Bromberg, J.F., M.H. Wrzeszczynska, G. Devgan, Y. Zhao, R.G. Pestell, C. Albanese, and J.E. Darnell Jr. 1999. The JAK-STAT pathway: summary of initial studies and recent advances. Stat3 as an oncogene. Cell. 98:295–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

