Mechanism of allosteric regulation of transglutaminase 2 by GTP
- PMID: 17179049
- PMCID: PMC1750866
- DOI: 10.1073/pnas.0609283103
Mechanism of allosteric regulation of transglutaminase 2 by GTP
Abstract
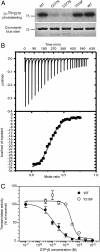
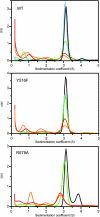
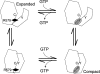
Allosteric regulation is a fundamental mechanism of biological control. Here, we investigated the allosteric mechanism by which GTP inhibits cross-linking activity of transglutaminase 2 (TG2), a multifunctional protein, with postulated roles in receptor signaling, extracellular matrix assembly, and apoptosis. Our findings indicate that at least two components are involved in functionally coupling the allosteric site and active center of TG2, namely (i) GTP binding to mask a conformationally destabilizing switch residue, Arg-579, and to facilitate interdomain interactions that promote adoption of a compact, catalytically inactive conformation and (ii) stabilization of the inactive conformation by an uncommon H bond between a cysteine (Cys-277, an active center residue) and a tyrosine (Tyr-516, a residue located on a loop of the beta-barrel 1 domain that harbors the GTP-binding site). Although not essential for GTP-mediated inhibition of cross-linking, this H bond enhances the rate of formation of the inactive conformer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Achyuthan KE, Greenberg CS. J Biol Chem. 1987;262:1901–1906. - PubMed
-
- Lorand L, Graham RM. Nat Rev Mol Cell Biol. 2003;4:140–157. - PubMed
-
- Lee KN, Birckbichler PJ, Patterson MK., Jr Biochem Biophys Res Commun. 1989;162:1370–1375. - PubMed
-
- Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM. Science. 1994;264:1593–1596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

