Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity
- PMID: 17179050
- PMCID: PMC1750916
- DOI: 10.1073/pnas.0609267103
Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity
Abstract
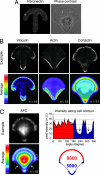
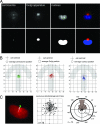
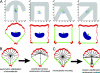
Control of the establishment of cell polarity is an essential function in tissue morphogenesis and renewal that depends on spatial cues provided by the extracellular environment. The molecular role of cell-cell or cell-extracellular matrix (ECM) contacts on the establishment of cell polarity has been well characterized. It has been hypothesized that the geometry of the cell adhesive microenvironment was directing cell surface polarization and internal organization. To define how the extracellular environment affects cell polarity, we analyzed the organization of individual cells plated on defined micropatterned substrates imposing cells to spread on various combinations of adhesive and nonadhesive areas. The reproducible normalization effect on overall cell compartmentalization enabled quantification of the spatial organization of the actin network and associated proteins, the spatial distribution of microtubules, and the positioning of nucleus, centrosome, and Golgi apparatus. By using specific micropatterns and statistical analysis of cell compartment positions, we demonstrated that ECM geometry determines the orientation of cell polarity axes. The nucleus-centrosome orientations were reproducibly directed toward cell adhesive edges. The anisotropy of the cell cortex in response to the adhesive conditions did not affect the centrosome positioning at the cell centroid. Based on the quantification of microtubule plus end distribution we propose a working model that accounts for that observation. We conclude that, in addition to molecular composition and mechanical properties, ECM geometry plays a key role in developmental processes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

