Functional organization of the Rpb5 subunit shared by the three yeast RNA polymerases
- PMID: 17179178
- PMCID: PMC1802627
- DOI: 10.1093/nar/gkl686
Functional organization of the Rpb5 subunit shared by the three yeast RNA polymerases
Abstract
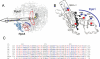
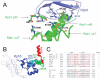
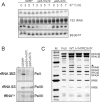
Rpb5, a subunit shared by the three yeast RNA polymerases, combines a eukaryotic N-terminal module with a globular C-end conserved in all non-bacterial enzymes. Conditional and lethal mutants of the moderately conserved eukaryotic module showed that its large N-terminal helix and a short motif at the end of the module are critical in vivo. Lethal or conditional mutants of the C-terminal globe altered the binding of Rpb5 to Rpb1-beta25/26 (prolonging the Bridge helix) and Rpb1-alpha44/47 (ahead of the Switch 1 loop and binding Rpb5 in a two-hybrid assay). The large intervening segment of Rpb1 is held across the DNA Cleft by Rpb9, consistent with the synergy observed for rpb5 mutants and rpb9Delta or its RNA polymerase I rpa12Delta counterpart. Rpb1-beta25/26, Rpb1-alpha44/45 and the Switch 1 loop were only found in Rpb5-containing polymerases, but the Bridge and Rpb1-alpha46/47 helix bundle were universally conserved. We conclude that the main function of the dual Rpb5-Rpb1 binding and the Rpb9-Rpb1 interaction is to hold the Bridge helix, the Rpb1-alpha44/47 helix bundle and the Switch 1 loop into a closely packed DNA-binding fold around the transcription bubble, in an organization shared by the two other nuclear RNA polymerases and by the archaeal and viral enzymes.
Figures






Similar articles
-
Activation of a chimeric Rpb5/RpoH subunit using library selection.PLoS One. 2014 Jan 29;9(1):e87485. doi: 10.1371/journal.pone.0087485. eCollection 2014. PLoS One. 2014. PMID: 24489922 Free PMC article.
-
Non-canonical DNA transcription enzymes and the conservation of two-barrel RNA polymerases.Nucleic Acids Res. 2010 Aug;38(14):4559-69. doi: 10.1093/nar/gkq201. Epub 2010 Mar 31. Nucleic Acids Res. 2010. PMID: 20360047 Free PMC article. Review.
-
Cloning, soluble expression, and purification of the RNA polymerase II subunit RPB5 from Saccharomyces cerevisiae.Bioengineered. 2015;6(1):62-6. doi: 10.1080/21655979.2014.1002301. Epub 2015 Jan 21. Bioengineered. 2015. PMID: 25551420 Free PMC article.
-
Mapping of Rpb3 and Rpb5 contact sites on two large subunits, Rpb1 and Rpb2, of the RNA polymerase II from fission yeast.Mol Gen Genet. 1998 Jul;259(1):123-9. doi: 10.1007/s004380050796. Mol Gen Genet. 1998. PMID: 9738888
-
Rpb5, a subunit shared by eukaryotic RNA polymerases, cooperates with prefoldin-like Bud27/URI.AIMS Genet. 2018 Feb 27;5(1):63-74. doi: 10.3934/genet.2018.1.74. eCollection 2018. AIMS Genet. 2018. PMID: 31435513 Free PMC article. Review.
Cited by
-
Activation of a chimeric Rpb5/RpoH subunit using library selection.PLoS One. 2014 Jan 29;9(1):e87485. doi: 10.1371/journal.pone.0087485. eCollection 2014. PLoS One. 2014. PMID: 24489922 Free PMC article.
-
Regulation of Eukaryotic RNAPs Activities by Phosphorylation.Front Mol Biosci. 2021 Jun 25;8:681865. doi: 10.3389/fmolb.2021.681865. eCollection 2021. Front Mol Biosci. 2021. PMID: 34250017 Free PMC article. Review.
-
The prefoldin bud27 mediates the assembly of the eukaryotic RNA polymerases in an rpb5-dependent manner.PLoS Genet. 2013;9(2):e1003297. doi: 10.1371/journal.pgen.1003297. Epub 2013 Feb 14. PLoS Genet. 2013. PMID: 23459708 Free PMC article.
-
Correct assembly of RNA polymerase II depends on the foot domain and is required for multiple steps of transcription in Saccharomyces cerevisiae.Mol Cell Biol. 2013 Sep;33(18):3611-26. doi: 10.1128/MCB.00262-13. Epub 2013 Jul 8. Mol Cell Biol. 2013. PMID: 23836886 Free PMC article.
-
Non-canonical DNA transcription enzymes and the conservation of two-barrel RNA polymerases.Nucleic Acids Res. 2010 Aug;38(14):4559-69. doi: 10.1093/nar/gkq201. Epub 2010 Mar 31. Nucleic Acids Res. 2010. PMID: 20360047 Free PMC article. Review.
References
-
- Werner F., Weinzierl R.O. A recombinant RNA polymerase II-like enzyme capable of promoter- specific transcription. Mol. Cell. 2002;10:635–646. - PubMed
-
- Pontier D., Yahubyan G., Vega D., Bulski A., Saez-Vasquez J., Hakimi M.A., Lerbs-Mache S., Colot V., Lagrange T. Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev. 2005;19:2030–2040. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

