Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB
- PMID: 17179947
- PMCID: PMC2013892
- DOI: 10.1038/sj.bjp.0706987
Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB
Abstract
Background and purpose: Extracts of Plumbago zeylanica containing suberosin exhibit anti-inflammatory activity. We purified suberosin from such extracts and studied its effects on a set of key regulatory events in the proliferation of human peripheral blood mononuclear cells (PBMC) stimulated by phytohemagglutinin (PHA).
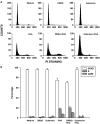
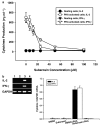
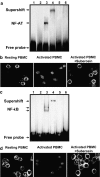
Experimental approach: Proliferation of PBMC in culture was measured by uptake of 3H-thymidine; production of cytokines and cyclins by Western blotting and RT-PCR. Transcription factors NF-AT and NF-kappaB were assayed by immunocytochemistry and EMSA.
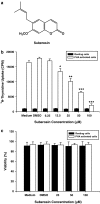
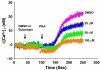
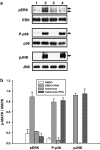
Key results: Suberosin suppressed PHA-induced PBMC proliferation and arrested cell cycle progression from the G1 transition to the S phase. Suberosin suppressed, in activated PBMC, transcripts of interleukin-2 (IL-2), interferon-gamma (IFN-gamma), and cyclins D3, E, A, and B. DNA binding activity and nuclear translocation of NF-AT and NF-kappaB induced by PHA were blocked by suberosin. Suberosin decreased the rise in intracellular Ca2+ concentration ([Ca2+]i) in PBMC stimulated with PHA. Suberosin did not affect phosphorylation of p38 and JNK but did reduce activation of ERK in PHA-treated PBMC. Pharmacological inhibitors of NF-kappaB, NF-AT, and ERK decreased expression of mRNA for the cyclins, IL-2, and IFN-gamma and cell proliferation in PBMC activated by PHA.
Conclusions and implications: The inhibitory effects of suberosin on PHA-induced PBMC proliferation, were mediated, at least in part, through reduction of [Ca2+]i, ERK, NF-AT, and NF-kappaB activation, and early gene expression in PBMC including cyclins and cytokines, and arrest of cell cycle progression in the cells. Our observations provide an explanation for the anti-inflammatory activity of P. zeylanica.
Figures


References
-
- Ahsan M, Zaman TA, Hasan CM, Ito C, Islam SK. Constituents and cytotoxicity of Zanthoxylum rhesta stem bark. Fitoterapia. 2000;71:697–700. - PubMed
-
- Ajchenbaum F, Ando K, DeCaprio JA, Griffin JD. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. - PubMed
-
- Arai K, Lee F, Miyajima A. Cytokines: Coordinators of immune and inflammatory response. Annu Rev Biochem. 1990;59:783–836. - PubMed
-
- Arnaud M, Crouin C, Deon C, Loyaux D, Bertoglio J. Phosphorylation of Grb2-associated binder 2 on serine 623 by ERK MAPK regulates its association with phosphatase SHP-2 and decreases STAT5 activation. J Immunol. 2004;173:3962–3971. - PubMed
-
- Buggins AGS, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NSB, et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-κB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–6030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

