Antitubercular nucleosides that inhibit siderophore biosynthesis: SAR of the glycosyl domain
- PMID: 17181146
- PMCID: PMC2526467
- DOI: 10.1021/jm061068d
Antitubercular nucleosides that inhibit siderophore biosynthesis: SAR of the glycosyl domain
Abstract
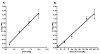
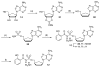
Tuberculosis is the leading cause of infectious disease mortality in the world by a bacterial pathogen. We previously demonstrated that a bisubstrate inhibitor of the adenylation enzyme MbtA, which is responsible for the second step of mycobactin biosynthesis, exhibited potent antitubercular activity. Here we systematically investigate the structure-activity relationships of the bisubstrate inhibitor glycosyl domain resulting in the identification of a carbocyclic analogue that possesses a KIapp value of 2.3 nM and MIC99 values of 1.56 microM against M. tuberculosis H37Rv. The SAR data suggest the intriguing possibility that the bisubstrate inhibitors utilize a transporter for entry across the mycobacterial cell envelope. Additionally, we report improved conditions for the expression of MbtA and biochemical analysis, demonstrating that MbtA follows a random sequential enzyme mechanism for the adenylation half-reaction.
Figures



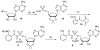


References
-
- World Health Organization. Fact sheet on tuberculosis. 2005. http://www.who.int/mediacentre/factsheets/fsl04/en/print.html. - PubMed
-
- CDC. Worldwide emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs. MMWR. 55(10):TK-TK. - PubMed
-
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. - PubMed
-
- Quadri LE. Assembly of aryl-capped siderophores by modular peptide synthetases and polyketide synthases. Mol Microbiol. 2000;37:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Miscellaneous

